
| ��Ӧʱ��/s | t1 | t2 | t3 | t4 | t5 |
| ����H2�������ʵ���/mol | 0.015 | 0.03 | 0.04 | 0.05 | 0.05 |
| 1 |
| x |
| 1 |
| 0.05mol |
| 10g-0.05mol��65g/mol |
| 10g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
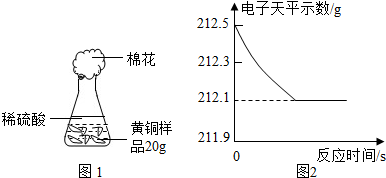
 Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�ij�о���ѧϰС���ȡ����Ʒ10g����������μ���9.8%��ϡ�������պò��ٲ�������Ϊֹ����Ӧ���������ɵ�����������������Һ��������ϵ��ͼ��ʾ���Լ��㣺
Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�ij�о���ѧϰС���ȡ����Ʒ10g����������μ���9.8%��ϡ�������պò��ٲ�������Ϊֹ����Ӧ���������ɵ�����������������Һ��������ϵ��ͼ��ʾ���Լ��㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ϡ�������� | ʣ��������� |
| ��һ�μ���10g | 4.7g |
| �ڶ��μ���10g | mg |
| �������10g | 2.1g |
| ���Ĵμ���10g | 1.2g |
| ������10g | 1.2g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�ij�о���ѧϰС���ȡ����Ʒ10g����������μ�ϡ�������պò��ٲ�������Ϊֹ����Ӧ���������ɵ����������������������ϵ��ͼ��ʾ������ͼʾ�ش����⣺
Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�ij�о���ѧϰС���ȡ����Ʒ10g����������μ�ϡ�������պò��ٲ�������Ϊֹ����Ӧ���������ɵ����������������������ϵ��ͼ��ʾ������ͼʾ�ش����⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com