
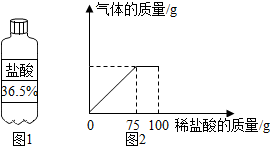
 ij����ѧϰС���ͬѧ��Ϊ�ⶨijʯ��ʯ��������������������������Ϊ7.3%��ϡ�������10gʯ��ʯ��Ʒ�У�������������������ϡ�����������ϵ��ͼ2��ʾ������ʯ��ʯ��Ʒ�е����ʲ�����Ԫ�أ�����ϡ���ᷴӦ����
ij����ѧϰС���ͬѧ��Ϊ�ⶨijʯ��ʯ��������������������������Ϊ7.3%��ϡ�������10gʯ��ʯ��Ʒ�У�������������������ϡ�����������ϵ��ͼ2��ʾ������ʯ��ʯ��Ʒ�е����ʲ�����Ԫ�أ�����ϡ���ᷴӦ�������� ��1��ϡ��ǰ�����ʵ��������䣬����Ҫ36.5%��Ũ���������Ϊx����100g��7.3%=36.5%x��x=20g��
��2����ͼ2��֪����Ӧ���������������ϡ���������Ϊ75g����HCl������Ϊ75g��7.3%�����û�ѧ����ʽ���ɼ�������뷴Ӧ��̼��Ƶ������������������Ʒ��̼��Ƶ�����������
��3����Ӧǰ��Ԫ�ص��������䣬��Ӧǰ���ձ��ڸ�Ԫ��û����ʧ���ʷ�Ӧ���ձ��и�Ԫ�ص��������䣮
��� �⣺��1��ϡ��ǰ�����ʵ��������䣬����Ҫ36.5%��Ũ���������Ϊx����100g��7.3%=36.5%x��x=20g�����20��
��2����ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73
x 75g��7.3%
$\frac{100}{73}=\frac{x}{75g��7.3%}$
x=7.5g
ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ��$\frac{7.5g}{10g}��100%$=75%
��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ75%��
��3����Ӧǰ��Ԫ�ص��������䣬��Ӧǰ���ձ��ڸ�Ԫ���������䣬�ʸ�Ԫ������Ϊ��7.5g��40%=3g��
���3g��
���� ������Ҫ���������ʵ�������������ؼ�������ݻ�ѧ����ʽ�ļ��㣬�ѶȲ������ʱҪϸ�ļ��㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼ��ʾΪij�ֲ��Ƽ����ƶ��桱˵�����һ���֣�ȡ1Ƭ�ƶ��棬����ʢ��30g������ձ��У����е�̼��Ƹ�ϡ����ǡ����ȫ��Ӧ�������ɷ��������Ӧ�����ձ������ʵ�������Ϊ11.34g�����㣺
��ͼ��ʾΪij�ֲ��Ƽ����ƶ��桱˵�����һ���֣�ȡ1Ƭ�ƶ��棬����ʢ��30g������ձ��У����е�̼��Ƹ�ϡ����ǡ����ȫ��Ӧ�������ɷ��������Ӧ�����ձ������ʵ�������Ϊ11.34g�����㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������ѧ��ѧ֪ʶ�ش�
������ѧ��ѧ֪ʶ�ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
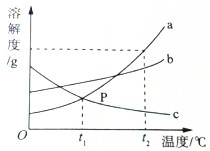 ͬѧ����ʵ����̽������a��b��c�ڲ�ͬ�¶�ʱ���ܽ�ȣ�����������ͼ��ʾ���������ʵ��ܽ�����ߣ����ͼ�ش�
ͬѧ����ʵ����̽������a��b��c�ڲ�ͬ�¶�ʱ���ܽ�ȣ�����������ͼ��ʾ���������ʵ��ܽ�����ߣ����ͼ�ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ᴿ | B�� | �ɱ����� | C�� | ʯ���� | D�� | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaCl��Ba��NO3��2��KOH | B�� | CaCl2��K2CO3��AgNO3 | ||
| C�� | NaNO3��Fe��NO3��3��HCL | D�� | H2SO4��Ba��NO3��2��K2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ȼúʹ�� | B�� | ��ɫ��̼���� | C�� | ����������� | D�� | �����ŷŷ�ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Fe3O4 | B�� | CO | C�� | Fe | D�� | CO2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com