
| ���ǵ��������ˣ� | ����ϡ������������ˣ� | �ռ���CO2����������� |
| 8 | 150 | 1.1 |
���� ��1��̼��������ᷴӦ���ɶ�����̼���壬������̼����ʹ����ʯ��ˮ����ǣ�
��2������̼��������ᷴӦ�Ļ�ѧ����ʽ�ͼ�����Ķ�����̼�����������ɼ�����õ�����̼��Ƶ�������Ȼ���������������ʽ���м��㼴�ɣ�
��� �⣺��1��̼��������ᷴӦ���ɶ�����̼���壬������̼����ʹ����ʯ��ˮ����ǣ��������ʯ��ˮ����ǣ�
��2����õ�����̼��Ƶ�����Ϊx��
CO2��������1.1L��2g/L=2.2g��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 2.2g
$\frac{100}{x}=\frac{44}{2.2g}$
x=5g��
���Ե�����̼��Ƶ�����������$\frac{5g}{8g}$��100%=62.5%��
���𣺵�����̼��Ƶ���������Ϊ62.5%��
���� �˽�̼���εļ��鷽��������ʽ����ĸ�ʽ�淶��ͬʱ��ȷ���뷽��ʽ�������Ӧ�����ʵ�������


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$C+2MgO���û���Ӧ�� | |
| B�� | 2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2�����ֽⷴӦ�� | |
| C�� | 2CO+O2$\frac{\underline{\;��ȼ\;}}{\;}$2CO2 �����Ϸ�Ӧ�� | |
| D�� | CH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$ CO2+2H2O���û���Ӧ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
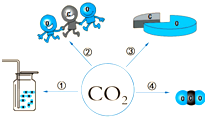 ģ�Ϳ��Է�ӳ���������˵ر�ʾ�����ͼ�Ƕ�����̼�Ļ�ѧʽ��CO2����������Ϣ��ģ�ͣ���д�����еĢ�������������Ϣ��
ģ�Ϳ��Է�ӳ���������˵ر�ʾ�����ͼ�Ƕ�����̼�Ļ�ѧʽ��CO2����������Ϣ��ģ�ͣ���д�����еĢ�������������Ϣ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���ᴿ���� | �������� | �����Լ� | �� �� �� �� |
| A | CO2 | CO | O2 | ��ȼ |
| B | KCl | K2CO3 | ���� | �ڻ�����м������������ٲ������ݣ������� |
| C | C�� | CuO | �����Լ� | ��������ȫ��Ӧ |
| D | Fe�� | Cu�� | ���� | �ڻ�����м������������ٲ������ݣ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ��Ŀ�� | ʵ�鷽�� | ||
| ʵ��� | ʵ��� | ||
| A�� | ̽��Zn��Fe��Cu �Ļ��ǿ�� |  |  |
| B�� | ̽���¶ȶԷ�Ӧ���ҳ̶ȵ�Ӱ�� |  |  |
| C�� | ̽�������Է�Ӧ������Ӱ�� |  |  |
| D�� | ̽��CO2 ���������ʺͻ�ѧ���� |  |  |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������Һ | B�� | Һ̬�Ȼ��� | C�� | Һ�� | D�� | ��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�Ϻӿ��о��꼶�п���Ӧ�Կ��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
2015��5��25�գ��佭��ijֽ��«έ�ֿⷢ�����֣�����ش�ľ�����ʧ��ijͬѧ��ֽ���ṩ������ܰ��ʾ������Ϊ�������
A��«έ�Ż�ʱ��ֻ����ɳ�����
B��«έ�ֿ���Ҫ�����Եķ���ʾ��־
C��«έ�ֿ�����Χ������֮��Ҫ�����㹻�ķ������
D��«έ�ֿ�Ҫ�����õ�ͨ���豸���뱸����������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com