�ҹ���һ�������ڿ�������������2003��3��1����ʵʩ�����й涨���ڿ����м�ȩ��HCHO���������ó���0.1mg/m3����ȩ�ĺ����ɸ������з�Ӧ�ⶨ��4KMnO4+5HCHO+6H2SO4=2K2SO4+4MnSO4+5CO2+11H2O
��ȡijװ������ڿ�����Ʒ500mL����������������Ϊ1.58��10-8��0.000 001 58%���ĸ��������Һ�����м���������������Һ��300g�������еļ�ȩǡ����ȫ��Ӧ��
��1����500mL�ÿ����м�ȩ��������
��2��ͨ������˵�����þ����ڿ����м�ȩ�����Ƿ���Ϲ��ҹ涨�ı���
��ϵʵ�ʣ���װ����ķ��ӣ����Բ���ʲô������ȥװ�����ͷų����ļ�ȩ���㻹��û�������ķ�����
�⣺��1�����ȩ������Ϊx
������������Ϊ1.58��10
-8�ĸ��������Һ300g�и�����ص�����=300g��1.58��10
-8=4.74��10
-6g
4KMnO
4+6H
2SO
4+5HCHO=2K
2SO
4+4MnSO
4+5CO
2+11H
2O
632 150
4.74��10
-6g x
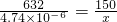
x=1.125��10
-6g=1.125��10
-3mg
��500mL�ÿ����м�ȩ������1.125��10
-3mg��
��2��500mL�ÿ����м�ȩ�ĺ���=
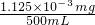
=2.25��10
-6mg/mL=2.25mg/m
3��0.1mg/m
3��
�ʴ�Ϊ���þ����ڿ����м�ȩ���������Ϲ��ҹ涨�ı���
ͨ�����ڿ�������ͨ�����Խ������ڿ������к����ʵĺ������Ӷ����ٴ������ʶ������Σ����������ʹ�û���̿������������ȥ��
�ʴ�Ϊ���������ڿ�����ͨ�����ڷ��û���̿��
��������1�����ݷ�Ӧ�Ļ�ѧ����ʽ���ɲμӷ�Ӧ�ĸ�����ص������ɼ���μӷ�Ӧ�ļ�ȩ��������
��2������500mL������������ȩ����������涨���ڿ����м�ȩ��HCHO���������ó���0.1mg/m
3���жԱȣ��ж����ڿ����м�ȩ�����Ƿꣻ�������ڼ�ȩ�������������ȥ�����飮
���������ݷ�Ӧ�Ļ�ѧ����ʽ���������ڿ����м�ȩ��������������ж��գ��ж����ڼ�ȩ�Ƿ�����

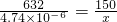 x=1.125��10-6g=1.125��10-3mg
x=1.125��10-6g=1.125��10-3mg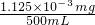 =2.25��10-6mg/mL=2.25mg/m3��0.1mg/m3��
=2.25��10-6mg/mL=2.25mg/m3��0.1mg/m3��

