
”¾ĢāÄæ”æijĶ¬Ń§“ŗÓĪ“ų»Ų¼øæéŹÆ»ŅŹÆѳʷ£®ĪŖ²ā¶Øѳʷ֊Ģ¼ĖįøʵÄŗ¬Įæ£¬Č”13.5gÕāÖÖŹÆ»ŅŹÆŹ¢ÓŚÉÕ±ÖŠ£¬ŌŁĻņÉÕ±ÖŠ¼ÓČė50gŅ»¶ØÖŹĮæ·ÖŹżµÄĻ”ŃĪĖį£ØŌÓÖŹ²»ČÜÓŚĖ®Ņ²²»ÓėŃĪĖį·“Ó¦£©£¬³ä·Ö·“Ó¦ŗóÉÕ±ÖŠĪļÖŹ×ÜÖŹĮæĪŖ59.1g£¬³ĘĮæŗóŌŁµĪ¼ÓÉŁĮæŃĪĖį²»ŌŁÓŠĘųÅŻ²śÉś£®ŹŌ¼ĘĖć£ŗ
£Ø1£©Éś³É¶žŃõ»ÆĢ¼µÄÖŹĮæĪŖ_______g£®
£Ø2£©³ĘĮæŗóŌŁµĪ¼ÓÉŁĮæŃĪĖįµÄÄæµÄŹĒ________£®
£Ø3£©ĖłČ”µÄŹÆ»ŅŹÆÖŠĢ¼ĖįøʵÄÖŹĮæ________£æ
”¾“š°ø”æ4.4g Č·ČĻĢ¼ĖįøĘŹĒ·ńĶźČ«·“Ó¦ 10g
”¾½āĪö”æ
£Ø1£©øł¾ŻÖŹĮæŹŲŗć¶ØĀÉŗĶĒ°ŗóµÄ×ÜÖŹĮæ±ä»ÆæÉÖŖ£¬Éś³ÉµÄ¶žŃõ»ÆĢ¼µÄÖŹĮæĪŖ13.5g+50g-59.1g=4.4g£»
£Ø2£©Č·ČĻĢ¼ĖįøĘŹĒ·ńĶźČ«·“Ó¦µÄ£¬øł¾ŻĻÖÓŠµÄĻÖĻóÓ¦øĆŹĒĢ¼ĖįøĘĶźČ«·“Ó¦£»
£Ø3£©½ā£ŗÉčĢ¼ĖįøʵÄÖŹĮæĪŖx£¬
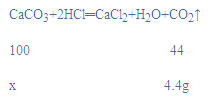
![]()
x=10g£¬
“š£ŗ£Ø1£©Éś³É¶žŃõ»ÆĢ¼µÄÖŹĮæĪŖ4.4g£»£Ø2£©³ĘĮæŗóŌŁµĪ¼ÓÉŁĮæŃĪĖįµÄÄæµÄŹĒČ·ČĻĢ¼ĖįøĘŹĒ·ńĶźČ«·“Ó¦£»£Ø3£©ĖłČ”µÄŹÆ»ŅŹÆÖŠĢ¼ĖįøʵÄÖŹĮæ10g”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĖ®¹ūÖŠŅņĪŖŗ¬ÓŠŅŅĖįŅŅõ„£Ø»ÆѧŹ½ĪŖC4H8O2£©£¬ŅņĪŖ»į³ŹĻÖ³öijÖÖĻćĪ¶£¬»Ų“šĻą¹ŲĪŹĢā£ŗ£ØŅŖĒ󊓳ö¼ĘĖć¹ż³Ģ£¬Ö»ÓŠ“š°ø²»øų·Ö£©
£Ø1£©ŅŅĖįŅŅõ„ÖŠĢ¼ŌŖĖŲŗĶŃõŌŖĖŲµÄÖŹĮæ±ČŹĒ¶ąÉŁ_____£æ
£Ø2£©ŅŅĖįŅŅõ„ÖŠĢ¼ŌŖĖŲµÄÖŹĮæ·ÖŹżŹĒ¶ąÉŁ________£æ£Ø¾«Č·µ½0.1%£©
£Ø3£©Čō22gŅŅĖįŅŅõ„ŌŚĢåÄŚ“śŠ»Ź±£¬ĘäĖłŗ¬µÄĢ¼ŌŖĖŲĶźČ«×Ŗ»ÆĪŖCO2£¬Ōņ²śÉśµÄCO2µÄÖŹĮæĪŖ¶ąÉŁ________£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ»ÆѧŹĒŌģø£ČĖĄąµÄæĘѧ£®
£ØŅ»£©Éś»īÖŠµÄ»Æѧ
£Ø1£©ŹŠ³”ÉĻŹ³ÓĆøĘʬ”¢²¹Ģś½“ÓĶÖŠµÄ”°øĘ”±”¢”°Ģś”±ÖøµÄŹĒ______£®
A£®·Ö×Ó B£®Ō×Ó C£®µ„ÖŹ D£®ŌŖĖŲ
£Ø2£©¹¹³É¼ÓµāŃĪÖŠÖ÷ŅŖ³É·ÖĀČ»ÆÄʵÄŅõĄė×ÓŹĒ______£ØŠ“Ąė×Ó·ūŗÅ£©
£Ø3£©¾»Ė®Ź±£¬ĪŖĮĖ¼ÓæģĖ®ÖŠŠüø”æÅĮ£³Į½µ£¬æÉŅŌĻņĖ®ÖŠ¼ÓČė______£ØĢīĪļÖŹĆū³Ę£©£®
£Ø4£©ÕōĮóĖ®²»ŅĖŃųÓć£¬ŹĒŅņĪŖÕōĮóĖ®ÖŠ¼øŗõ²»ŗ¬_________£ØĢī×ÖÄøŠņŗÅ£©£®
A£®ŃõŌŖĖŲ B. ŃõŌ×Ó C. Ńõ·Ö×Ó D. ŃõĄė×Ó
£Ø¶ž£©Å©ŅµÉĻ»Æ·ŹµÄŹ¹ÓĆĢ½¾æ
£Ø5£©ĻĀĶ¼ŹĒij»Æ·ŹĢ¼ĖįĒāļ§°ü×°“üÉĻµÄÉĢ±ź£¬ÉĢ±źÉĻæÉÖŖĢ¼ĖįĒāļ§¾ßÓŠµÄŠŌÖŹŹĒ__£ØĢī×ÖÄø£©£®
A£®Ņ×ČÜÓŚĖ® B£®ÓŠ»Ó·¢ŠŌ C£®ŹÜČČŅ×·Ö½ā

£Ø6£©ÕāÖֻƷŹŗ¬µŖĮæŹĒ·ń“ļµ½16%£¬»Æ·ŹÖŠĢ¼ĖįĒāļ§µÄŗ¬ĮæŹĒ¶ąÉŁ£æ“ų×ÅÕāŠ©ĪŹĢā£¬ŠĖȤŠ”×éµÄĶ¬Ń§Č”ĮĖŅ»Š©»Æ·ŹŃłĘ·£¬½ųČėŹµŃéŹŅ£®
£Ø²éÕŅ׏ĮĻ£©¢Ł¼īŹÆ»ŅÄܹ»ĪüŹÕĖ®ŗĶCO2£¬µ«ŹĒ²»ĪüŹÕNH3£®
¢ŚÅØĮņĖįÄÜĪüŹÕNH3µ«ŹĒ²»ĪüŹÕCO2£®
¢Ū°±ĘųČÜÓŚĖ®ŠĪ³É°±Ė®
£ØŠŌÖŹĢ½¾æ£©øĆĶ¬Ń§Éč¼ĘĮĖČēĻĀµÄŹµŃé×°ÖĆ£ŗ
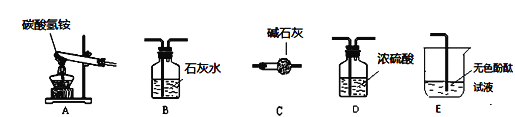
¢ŁÓĆA×°ÖĆøųĢ¼ĖįĒāļ§¼ÓČČ£¬×°Ņ©Ę·Ē°£¬±ŲŠė½ųŠŠµÄŅ»²½²Ł×÷ŹĒ________£®
¢ŚČ”ŹŹĮæĢ¼ĖįĒāļ§¼ÓČėŹŌ¹Ü£¬Į¬½ÓA”¢C”¢E×°ÖĆ£¬¼ÓČČ£¬EÖŠµÄĻÖĻóŹĒ_____£®
¢ŪĮ¬½ÓA”¢B×°ÖĆ£¬¼ĢŠų¼ÓČČ£¬¹Ū²ģµ½ŹŌ¹ÜæŚµÄĻÖĻóŹĒ____£¬BÖŠµÄĻÖĻóŹĒ____£®
¢ÜĢ¼ĖįĒāļ§ŌŚŹÜČČŹ±·¢Éś·“Ó¦µÄ»ÆѧŹ½±ķ“ļŹ½ŹĒ____________£®
£Øŗ¬Įæ·ÖĪö£©½«×°ÖĆA”¢C”¢DŅĄ“ĪĮ¬½Ó£¬¼ÓČė20g»Æ·ŹŃłĘ·£¬¼ÓČČÖĮAÖŠ¹ĢĢåĶźČ«ĻūŹ§£®Ėż³ĘĮæ×°ÖĆDµÄÖŹĮæČē±ķ£ŗ
ŹµŃéĒ°D×°ÖƵÄÖŹĮæ | 149g |
ŹµŃéŗóD×°ÖƵÄÖŹĮæ | 152.4g |
¢ŻÓÉ“Ė·ÖĪöµĆÖŖ£ŗ·“Ó¦ÖŠ²śÉś°±ĘųµÄÖŹĮæĪŖ_______g£®
¢ŽĶعż·“Ó¦µÄ±ķ“ļŹ½æÉŅŌÖŖµĄ£ŗ°±ĘųÖŠµŖŌŖĖŲČ«²æĄ“×ŌÓŚĢ¼ĖįĒāļ§£Ø¼ŁÉčŌÓÖŹÖŠ²»ŗ¬µŖŌŖĖŲ£©£¬Ēė¼ĘĖć“Ė»Æ·Źŗ¬µŖŌŖĖŲÖŹĮæ·ÖŹżĪŖ_______£®
¢ßĒė¼ĘĖć“Ė»Æ·ŹÖŠĢ¼ĖįĒāļ§µÄ“æ¶Č________£®
¢ą______Ē§æĖµÄÄņĖŲ[CO(NH2)2]ŗĶÕā“ü»Æ·ŹµÄŗ¬µŖŌŖĖŲÖŹĮæĻąµČ£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æijKClO3ŗĶMnO2×é³ÉµÄ»ģŗĻĪļÖŠ¼ŲŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ30%£¬½«13.0gøĆ»ģŗĻĪļ¼ÓČČÖĮ¹ĢĢåÖŹĮæ²»ŌŁøı䣬ĻĀĮŠÓŠ¹ŲĖµ·Ø“ķĪóµÄŹĒ
A. ¼ÓČČŗó¹ĢĢåÖŠ¼ŲŌŖĖŲµÄÖŹĮæ·ÖŹżŌö¼Ó B. ¹²Éś³É3.2gŃõĘų
C. ¼ÓČČŗó£¬Ź£Óą¹ĢĢåµÄÖŹĮæĪŖ8.2g D. Ō»ģŗĻĪļÖŠMnO2µÄÖŹĮæĪŖ0.75g
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”ææÕĘųÖŠŃõĘųŗ¬Įæ²ā¶ØµÄŌŁČĻŹ¶
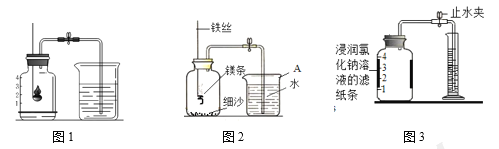
¢ń. ÓĆŗģĮײā¶ØæÕĘųÖŠŃõĘųµÄŗ¬Į攣
£Ø1£©ĒėŠ“³öŗģĮ×ŌŚæÕĘųÖŠČ¼ÉյĻÆѧ±ķ“ļŹ½________”£
£Ø2£©ČēĶ¼1ĖłŹ¾½ųŠŠŹµŃ飬 ²āµĆæÕĘųÖŠŃõĘųµÄŗ¬ĮæŠ”ÓŚ1ØM5£¬æÉÄܵÄŌŅņŹĒ ____£ØĢīŠņŗÅ£©”£
A£®Č¼ÉÕ³×ÉģČė¼ÆĘųĘæĢ«Āż B£®ŗģĮ×Įæ²»×ć
C£® ŹµŃéÖŠ·¢ĻÖµ¼¹ÜæŚÓŠĘųÅŻĆ°³ö D£®×°ÖĆĀ©Ęų
¢ņ. ÓĆĆ¾Ģõ²ā¶ØæÕĘųÖŠŃõĘųµÄŗ¬Į攣
£Ø3£©ĒėŠ“³öĆ¾ĢõŌŚŃõĘųÖŠČ¼ÉյĻÆѧ±ķ“ļŹ½_____________”£
£Ø4£©Ä³Ķ¬Ń§ĄūÓĆĶ¼2ŹµŃé²ā¶ØæÕĘųÖŠŃõĘųŗ¬ĮæŹ±·¢ĻÖ£¬²śĪļÖŠ»¹³öĻÖÉŁŠķ»ĘÉ«¹ĢĢ唣
ŅŃÖŖ£ŗĆ¾ÄÜÓėµŖĘų·“Ӧɜ³Éµ»ĘÉ«µÄµŖ»ÆĆ¾£ØMg3N2£©¹ĢĢ唣 µ«ŌŚæÕĘųÖŠČ¼ÉÕĆ¾ĢõŹ±ŗÜÄŃ¹Ū²ģµ½Éś³ÉĪļÖŠÓŠµ»ĘÉ«¹ĢĢ壬ŌŅņŹĒ_________”£ŌŚ²Ł×÷¹ę·¶µÄĒéæöĻĀ£¬øĆĶ¬Ń§ŹµŃéĖł²āµĆµÄŃõĘųĢå»ż·ÖŹż______1/5£ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©”£
¢ó. ÓĆĢś·Ū²ā¶ØæÕĘųÖŠŃõĘųµÄŗ¬Į攣
£Ø5£©ŅŃÖŖĢś³£ĪĀĻĀÓėæÕĘųÖŠµÄŃõĘų”¢Ė®·“Ӧɜ³ÉĢśŠā£ØÖ÷ŅŖ³É·ÖŹĒFe2O3©qxH2O£©”£ŹŌŠ“³öøĆ·“Ó¦µÄ»Æѧ±ķ“ļŹ½________”£
£Ø6£©Ä³Ķ¬Ń§øł¾ŻĢśÉśŠāµÄŌĄķ£¬ÓĆĢś·Ū²ā¶ØæÕĘųÖŠŃõĘųµÄŗ¬Į棬Éč¼ĘĮĖČēĶ¼ŹµŃ飬8·ÖÖÓŗó²āµĆŹż¾ŻČē±ķ£ŗ
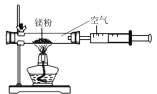
ŹµŃéĒ°µÄĢå»ż | ŹµŃéŗóµÄĢå»ż | |
¼ÆĘųĘæÄŚæÕĘų | ĮæĶ²ÄŚĖ® | ĮæĶ²ÄŚŹ£ÓąĖ® |
250mL | 180.0mL | 129.5mL |
ŹµŃ鏱øĆĶ¬Ń§²»ŹĒ½«Ģś·Ū¶ŃŌŚĘæµ×£¬¶ųŹĒ½«Ģś·ŪČöŌŚ½žČóĮĖĀČ»ÆÄĘČÜŅŗµÄĀĖÖ½ĢõÉĻ£¬ŌŁ°ŃøĆĀĖÖ½ĢõĢłŌŚ¹ćæŚĘæÄŚ²ą£¬ĘäÄæµÄŹĒ________”£øł¾Ż±ķÖŠŹż¾Ż¼ĘĖćµĆ³öæÕĘųÖŠŃõĘųµÄĢå»ż·ÖŹżĪŖ________£Ø½į¹ū±£Įōµ½0.1%£©”£ÓėÓĆŗģĮ×Č¼Éյķ½·ØĻą±Č£¬ÓĆøĆ·½·Ø²ā¶ØæÕĘųÖŠŃõĘųµÄŗ¬ĮæµÄÖ÷ŅŖÓŵćŅ»ŹĒƻӊĪŪČ¾£¬¶žŹĒ ____________”£
¢ō.ĶŲÕ¹Ó¦ÓĆ
£Ø7£©Č”2.4gĆ¾ĢõŌŚO2ŗĶN2µÄ»ģŗĻĘųĢåÖŠĶźČ«Č¼ÉÕ£¬ĖłµĆ¹ĢĢåÖŹĮæĪŖag,£¬ŌņaµÄȔֵ·¶Ī§ŹĒ________g£¼a g£¼4g£Ø½į¹ū±£Įōµ½0. 1g£©”£
£Ø8£©ŅŃÖŖ£ŗFe2O3¹ĢĢåŌŚŅ»¶ØµÄøßĪĀĻĀÄÜ·Ö½āĪŖŅ»ÖÖø“ŌÓµÄĢśµÄŃõ»ÆĪļŗĶŅ»ÖÖĘųĢ唣 øĆĘųĢåæÉŅŌÓĆ___________Ą“¼ģŃ锣ĻÖ½«64.0gFe2O3¹ĢĢå¼ÓČȵ½øĆĪĀ¶ČĻĀ£¬·¢ĻÖ¹ĢĢåÖŹĮæ¼õÉŁĮĖ3.2gŗóÖŹĮæ²»ŌŁøı䔣Ēė»Ų“š£ŗ64.0gFe2O3¹ĢĢåÖŠŗ¬ÓŠĢśŌŖĖŲ_______g£»øĆĪĀ¶ČĻĀø“ŌÓµÄĢśµÄŃõ»ÆĪļµÄ»ÆѧŹ½ĪŖ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÕįĢĒŹĒ³£ÓƵĵ÷Ī¶Ę·”£¹¤ŅµÖʱøÕįĢĒµÄĮ÷³ĢČēĻĀĶ¼ĖłŹ¾”£
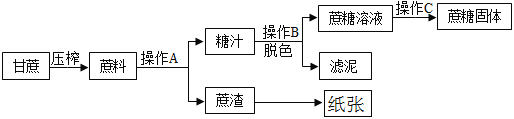
£Ø1£©²Ł×÷AĆū³ĘŹĒ______£¬ŹµŃéŹŅ½ųŠŠ“Ė²Ł×÷Ź±Ź¹ÓĆ²£Į§°ōµÄ×÷ÓĆŹĒ_____£»
£Ø2£©ĢĒÖ³Ź×Ų»ĘÉ«£¬¹¤ŅµÉĻæɼÓČė_________¶ŌĢĒÖ½ųŠŠĶŃÉ«“¦Ąķ”£
£Ø3£©Čē²Ł×÷CŹĒ¼ÓČČÕō·¢£¬Ņ»¶ĪŹ±¼äŗóÕō·¢Ćóµ×²ææÉÄÜ»įµĆµ½ŗŚÉ«¹ĢĢ壬ĖµĆ÷ÕįĢĒµÄ×é³ÉÖŠŗ¬ÓŠ_________£»
£Ø4£©ÕįŌüŌģÖ½¹ż³ĢÖŠ³£ÓƵÄĘÆ°×¼ĮŹĒ¶žŃõ»ÆĀČ£ØClO2£©”£60”ꏱÓĆĀČĖį¼ŲŗĶ²ŻĖį£ØH2C2O4)¼“æÉÉś³É¶žŃõ»ÆĀČ”¢¶žŃõ»ÆĢ¼”¢Ģ¼Ėį¼ŲŗĶĖ®£¬·“Ó¦µÄ·ūŗűķ“ļŹ½ĪŖ£ŗ___________£»
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¹żŃõ»ÆĒāČÜŅŗŌŚ¶žŃõ»ÆĆĢ×÷“߻ƼĮµÄĢõ¼žĻĀÄÜŃøĖŁ·Ö½ā£¬·ÖŅŗĀ©¶·æÉŅŌĶعżµ÷½Ś»īČūæŲÖĘŅŗĢåµÄµĪ¼ÓĖŁ¶Č£¬Ēėøł¾ŻČēĶ¼»Ų“šĪŹĢā£ŗ
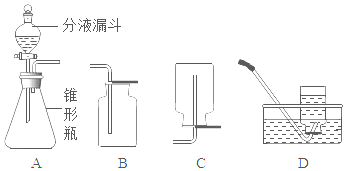
£Ø1£©·ÖŅŗĀ©¶·ÖŠÓ¦·ÅČėµÄĪļÖŹŹĒ________£¬×¶ŠĪĘæÖŠÓ¦·ÅČėµÄĪļÖŹŹĒ_________”£
£Ø2£©Š“³öÓĆøĆ·½·ØÖĘČ”ŃõĘųµÄ»Æѧ·“Ó¦»Æѧ·½³ĢŹ½_______”£ŅŖŹÕ¼ÆŅ»Ęæ“æ¾»µÄŃõĘų£¬Ó¦Ń”Ōń×°ÖĆ___£ØĢī×ÖÄø£©
£Ø3£©Ä³Ķ¬Ń§¹Ū²ģµ½×¶ŠĪĘæÄŚÓŠ“óĮæĘųÅŻŹ±£¬æŖŹ¼ÓĆB×°ÖĆŹÕ¼ÆŃõĘų£¬¹żŅ»¶ĪŹ±¼äŗóÓĆ“ų»šŠĒµÄľĢõÉģČėĘææŚ”¢ĘæÖŠŗĶĘæµ×£¬¶¼Ä©¼ūľĢõø“Č¼£®ĘäŌŅņŹĒ_________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ2015ÄźĪŅ¹śÅ®æĘѧ¼ŅĶĄßĻßĻ»ńµĆŵ±“¶ūÉśĄķѧ»ņŅ½Ń§½±£¬Ėż·¢ĻÖĒąŻļĖŲ£ØĒąŻļĖŲµÄ»ÆѧŹ½£ŗC15H22O5£©ŹĒŅ»ÖÖÖĪĮĘÅ°¼²µÄŅ©Īļ”£Ēė¼ĘĖć£ŗ
£Ø1£©ĒąŻļĖŲµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ______£»
£Ø2£©Ņ»øöĒąŻļĖŲ·Ö×ÓÖŠŗ¬ÓŠ____øöŌ×Ó£¬ĒąŻļĖŲ·Ö×ÓÖŠĢ¼”¢Ēā”¢ŃõČżÖÖŌŖĖŲµÄŌ×ÓøöŹż±ČĪŖ_____£»
£Ø3£©ĒąŻļĖŲÖŠø÷ŌŖĖŲµÄÖŹĮæ±ČĪŖ______£»
£Ø4£©ĒąŻļĖŲÖŠĢ¼ŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ______£Ø¼ĘĖć½į¹ū±£ĮōŅ»Ī»Š”Źż£©£»
£Ø5£©100g ĒąŻļĖŲÖŠŗ¬Ģ¼ŌŖĖŲµÄÖŹĮæĪŖ______g”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĶź³ÉĻĀĮŠŹµŃéĢā
£ØŹµŃéŅ»£©½įŗĻ¾»»Æ»ĘÄąĖ®µÄ»ī¶Æ¾ŃéŗĶ¼Ó¹¤×ŌĄ“Ė®µÄŌĄķ£¬Öø³öĻĀĮŠŌÓÖŹµÄ³żČ„·½·Ø£ØĢī·½·ØĆū³Ę£©
Ė®ÖŠŌÓÖŹÖÖĄą | ³żČ„ŌÓÖŹµÄ·½·ØĆū³Ę |
æÅĮ£½Ļ“óµÄ²»ČÜŠŌŌÓÖŹ | ____ |
æÅĮ£½ĻŠ”µÄ²»ČÜŠŌŌÓÖŹ | ____ |
æÉČÜŠŌŌÓÖŹ | ____ |
£ØŹµŃ鶞£©ŹµŃéŹŅÓūÅäÖĘŅ»¶ØÖŹĮæ·ÖŹżµÄŹ³ŃĪČÜŅŗ”£ĒėĶź³ÉĻĀĮŠĪŹĢā£ŗ
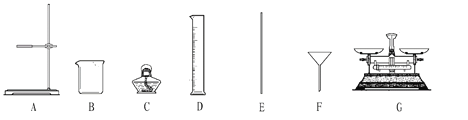
£Ø1£©BŅĒĘ÷µÄĆū³ĘŹĒ_______£¬DŅĒĘ÷µÄĆū³ĘŹĒ_____”£
£Ø2£©Ķź³É±¾ŹµŃ飬ĖłŠčŅĒĘ÷³żB”¢EĶā£¬»¹±ŲŠėŃ”ÓĆÉĻŹöŅĒĘ÷ÖŠµÄ_______£ØĢīŅĒĘ÷±ąŗÅ£©£¬ŅĒĘ÷EµÄ×÷ÓĆŹĒ_______”£
£Ø3£©ÅäÖĘµÄ²½ÖčæÉŅŌøÅĄØĪŖ¼ĘĖć”¢³ĘĮæŗĶĮæČ””¢_______”¢×°Ę攣
£Ø4£©Čē¹ūĮæČ”Ė®µÄĢå»żŹ±£¬²Ł×÷ÕߵďÓĻߏĒŃöŹÓ£¬ŌņĖłÅäČÜŅŗµÄÖŹĮæ·ÖŹż½«_______£ØĢī”°Ę«“ó”±»ņ”°Ę«Š””±»ņ”°²»±ä”±£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com