

 ×100%¼°±äŹ½ÄÜĮé»īµÄÓ¦ÓĆ£®øł¾Ż»Æѧ·½³ĢŹ½¼ĘĖć“ųČėµÄŹż¾Ż±ŲŠėŹĒ“æĪļÖŹµÄÖŹĮ棬Čē¹ūĢāŅāÖŠĢį¹©µÄŹż¾ŻŹĒ»ģŗĻĪļµÄÖŹĮ棬±ŲŠė½ųŠŠ×Ŗ»Æ³É“æĪļÖŹµÄÖŹĮ森
×100%¼°±äŹ½ÄÜĮé»īµÄÓ¦ÓĆ£®øł¾Ż»Æѧ·½³ĢŹ½¼ĘĖć“ųČėµÄŹż¾Ż±ŲŠėŹĒ“æĪļÖŹµÄÖŹĮ棬Čē¹ūĢāŅāÖŠĢį¹©µÄŹż¾ŻŹĒ»ģŗĻĪļµÄÖŹĮ棬±ŲŠė½ųŠŠ×Ŗ»Æ³É“æĪļÖŹµÄÖŹĮ森 =66.9mL£¬¹Ź“š°øĪŖ£ŗ66.9mL
=66.9mL£¬¹Ź“š°øĪŖ£ŗ66.9mL =
=
 =0.4g
=0.4g =
=
 =11.2g
=11.2g  =
=
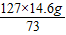 =25.4g
=25.4g ×100%=97.4%
×100%=97.4% ×100%=25.4%
×100%=25.4% =
= ¢Ū97.4% ¢Ü25.4%
¢Ū97.4% ¢Ü25.4% 2Fe+3CO2
2Fe+3CO2 =
=
 =7.1Ķņ¶Ö
=7.1Ķņ¶Ö

 æģĄÖ5¼Ó2½š¾ķĻµĮŠ“š°ø
æģĄÖ5¼Ó2½š¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2008ÄźŗŚĮś½Ź”¹ž¶ū±õŹŠÖŠæ¼»ÆŃ§Ä£ÄāŹŌ¾ķ£ØĮł£©£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
| A | B | C | D | |
| Ēų·ÖµÄĪļÖŹ | »ĘĶŗĶ»Ę½š | ŃņĆ«ŗĶµÓĀŚ | Ģ¼Ėį¼ŲŗĶĀČ»Æļ§ | Ź³ŃĪŗĶ°×ĢĒ |
| µŚŅ»·½°ø | ¹Ū²ģŃÕÉ« | ¹Ū²ģÉ«Ōó | Ȕѳ£¬Čܽā£¬·Ö±šµĪ¼ÓĪŽÉ«·ÓĢŖŹŌŅŗ | ¹Ū²ģŠĪד |
| µŚ¶ž·½°ø | Ȕѳ£¬·Ö±šµĪ¼ÓĻ”ŃĪĖį | Ȕѳ£¬·Ö±šµćČ¼£¬ĪÅČ¼ÉÕŹ±µÄĘųĪ¶ | Ȕѳ£¬·Ö±š¼ÓČėŹģŹÆ»Ņ£¬ŃŠÄ„£¬ĪÅĘųĪ¶ | Ę·³¢Ī¶µĄ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2008ÄźŗŚĮś½Ź”¹ž¶ū±õŹŠĖɱ±ĒųÖŠæ¼»ÆѧŅ»Ä£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
| A | B | C | D | |
| Ēų·ÖµÄĪļÖŹ | »ĘĶŗĶ»Ę½š | ŃņĆ«ŗĶµÓĀŚ | Ģ¼Ėį¼ŲŗĶĀČ»Æļ§ | Ź³ŃĪŗĶ°×ĢĒ |
| µŚŅ»·½°ø | ¹Ū²ģŃÕÉ« | ¹Ū²ģÉ«Ōó | Ȕѳ£¬Čܽā£¬·Ö±šµĪ¼ÓĪŽÉ«·ÓĢŖŹŌŅŗ | ¹Ū²ģŠĪד |
| µŚ¶ž·½°ø | Ȕѳ£¬·Ö±šµĪ¼ÓĻ”ŃĪĖį | Ȕѳ£¬·Ö±šµćČ¼£¬ĪÅČ¼ÉÕŹ±µÄĘųĪ¶ | Ȕѳ£¬·Ö±š¼ÓČėŹģŹÆ»Ņ£¬ŃŠÄ„£¬ĪÅĘųĪ¶ | Ę·³¢Ī¶µĄ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźŗŚĮś½Ź”¹ž¶ū±õŹŠÄĻøŚĒųÖŠæ¼»ÆѧŅ»Ä£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźŗŚĮś½Ź”¹ž¶ū±õŹŠÄĻøŚĒųÖŠæ¼»ÆѧŅ»Ä£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźŗŚĮś½Ź”¹ž¶ū±õŹŠÖŠæ¼»ÆŃ§Ä£ÄāŹŌ¾ķ£ØČż£©£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com