
| 1 | 10 |
| 1 |
| 10 |
| ���� |
| 1 |
| 10 |
| 1 |
| 10 |
| 1 |
| 10 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
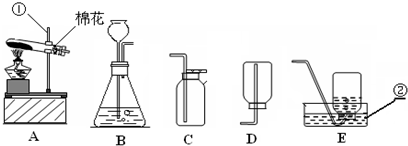
 ��������ʵ��װ��ͼ���ش��й����⣺
��������ʵ��װ��ͼ���ش��й����⣺| �������� |
| �������� |
�鿴�𰸺ͽ���>>
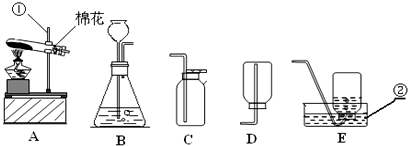
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
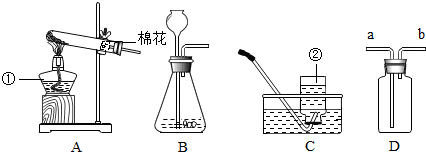
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ������ѧ���꼶��һѧ��9�¿���ѧ�Ծ����������� ���ͣ�������
��������ʵ��װ��ͼ���ش��й����⣺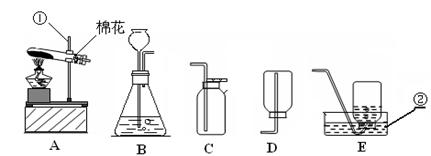
��1��д��������������ƣ��� ��
��2�������A��E���װ����ȡ������С��˵������A��C��ϣ�������__________________��д����Ӧ�����ֻ���ű���ʽΪ ________________________ ���Թܿڷ�һ������Ŀ���� _____________________�����ù���������Һ�����������ȡ��������ѡ�õķ���װ���� _______�����ţ������ж��������ڷ�Ӧ����________���á�
��3�����ʵ��Ĺ������Թܳ��������ѣ����������Ϊ��_____________ ____���� �����µģ�����д���㣩��
��4��������ռ�������������˿ȼ��ʵ��ʱ��Ԥ���ڼ���ƿ�ײ�������ˮ��Ŀ���� ��
��5��ʵ�����ø����������ȡ��������̽������������������Լ�Ǹ������������1/10���ش��������⣬
�����и������32 g����Լ�����������������Ƕ��٣�д��������̣�
����Щ�����൱�ڶ������Ŀ����������е����������ȡ���������������ڸ��������ܶ�Ϊ1.43 g/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ʵ��װ��ͼ���ش��й����⣺
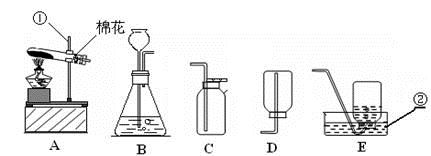
��1��д��������������ƣ��� ��
��2�������A��E���װ����ȡ������С��˵������A��C��ϣ�������__________________��д����Ӧ�����ֻ���ű���ʽΪ ________________________ ���Թܿڷ�һ������Ŀ���� _____________________�����ù���������Һ�����������ȡ��������ѡ�õķ���װ���� _______�����ţ������ж��������ڷ�Ӧ����________���á�
��3�����ʵ��Ĺ������Թܳ��������ѣ����������Ϊ��_____________ ____���� �����µģ�����д���㣩��
��4��������ռ�������������˿ȼ��ʵ��ʱ��Ԥ���ڼ���ƿ�ײ�������ˮ��Ŀ���� ��
��5��ʵ�����ø����������ȡ��������̽������������������Լ�Ǹ������������1/10���ش��������⣬
�����и������32 g����Լ�����������������Ƕ��٣�д��������̣�
����Щ�����൱�ڶ������Ŀ����������е����������ȡ���������������ڸ��������ܶ�Ϊ1.43 g/L��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com