
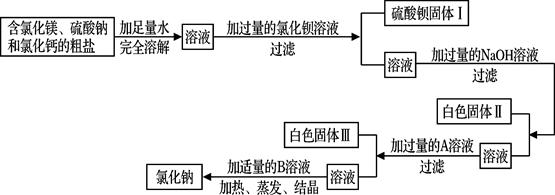
(8·Ö)ÉĒĶ·ŹĒĆĄĄöµÄŗ£±õ³ĒŹŠ£¬ŗ£ŃóŹĒ·įø»µÄ»Æѧ׏Ō“±¦æā£¬ĶعżĮĄÉ¹ŗ£Ė®æÉŅŌµĆµ½ŗ¬ÓŠ½Ļ¶ąŌÓÖŹµÄ“ÖŃĪ”£
(1)ŌŚ×ö“ÖŃĪ³õ²½Ģį“æµÄŹµŃ鏱£¬ŅŖ½ųŠŠČēĻĀĶ¼ĖłŹ¾µÄŹµŃé²Ł×÷”£

¢Ł²Ł×÷BÖŠ²£Į§°ōµÄ×÷ÓĆŹĒ
¢Ś²Ł×÷C֊擵½ Ź±£¬Ķ£Ö¹¼ÓČČ”£
(2)ÓĆĢģĘ½³ĘĮæŅ»¶ØĮæµÄŗ¬ĀČ»ÆĆ¾”¢ĮņĖįÄĘŗĶĀČ»ÆøʵēÖŃĪ£¬²¢Éč¼ĘĮĖČēĻĀĮ÷³Ģ½ųŠŠ³żŌÓĢį“棬½ųŅ»²½µĆµ½½Ļ“æ¾»µÄĀČ»ÆÄĘ¹ĢĢ唣

øł¾ŻĮ÷³ĢĶ¼»Ų“šĻĀĮŠĪŹĢā£ŗ
¢Ł”°¼ÓČė¹żĮæµÄAČÜŅŗ”±£¬ĘäÖŠAŹŌ¼ĮŹĒ £»
¢ŚŠ“³ö·“Ó¦Į÷³ĢÖŠ¼ÓČėĀČ»Æ±µČÜŅŗÉś³ÉÉś³ÉĮņĖį±µµÄ»Æѧ·“Ó¦·½³ĢŹ½ £»
¢Ū°×É«¹ĢĢå¢ņµÄ³É·ÖŹĒMg(OH)2£¬°×É«¹ĢĢå¢óµÄ³É·ÖŹĒ ŗĶ £»
¢Ü¼ÓČėŹŹĮæBČÜŅŗ£¬BČÜŅŗŹĒ ”£
(1)ŅżĮ÷£»½Ļ¶ą¹ĢĢå (2)¢ŁNa2CO3 ¢ŚBaCl2+ Na2SO4=BaSO4”ż+2NaCl (2·Ö)
¢ŪCaCO3£»BaCO3 ¢ÜĻ”ŃĪĖį
½āĪö:£Ø1£©¢Ł²£Į§°ōµÄ×÷ÓĆŹĒŅżĮ÷£®¢ŚÕō·¢²Ł×÷֊擵½Õō·¢ĆóÖŠ³öĻÖ½Ļ¶ą¹ĢĢåŹ±£¬Ķ£Ö¹¼ÓČČ£¬ÓĆÓąČČ°ŃĖ®·ÖÕōøÉ£®
£Ø2£©³żČ„“ÖŃĪÖŠµÄĀČ»ÆøĘ£¬ĀČ»ÆĆ¾ŗĶĮņĖįÄĘ£¬³żøĘĄė×ÓÓĆĢ¼ĖįÄĘ£¬³żČ„Ć¾Ąė×ÓÓĆĒāŃõ»ÆÄĘ£¬³żČ„ĮņĖįøłÓĆĀČ»Æ±µ£¬ŅŖ×¢Ņā¼ÓČėŹŌ¼ĮµÄĖ³ŠņŗĶĮ棬ŗó¼ÓČėµÄŹŌ¼ĮŅŖ°ŃĒ°Ćę¼ÓČėµÄ¹żĮæŹŌ¼ĮŅ²³żČ„£»
¢Ł”°¼ÓČė¹żĮæµÄAČÜŅŗ”±µÄÄæµÄŹĒ³żČ„ĀČ»ÆøĘŗĶĀČ»Æ±µ£¬ĖłŅŌAŹŌ¼ĮŹĒ Na2CO3£»
¢Ś°×É«¹ĢĢå¢ņµÄ³É·ÖŹĒ¼ÓČėĒāŃõ»ÆÄĘŗóÉś³ÉµÄ£¬¹ŹŹĒ Mg£ØOH£©2³Įµķ£»°×É«¹ĢĢå¢óµÄ³É·ÖŹĒĀČ»ÆøĘŗĶĀČ»Æ±µÓė¼ÓČėĢ¼ĖįÄĘÉś³ÉµÄ£¬¹Ź³ĮµķŹĒCaCO3 BaCO3Į½ÖÖĪļÖŹ£»
¢Ū¼ÓČėŹŹĮæBČÜŅŗŹ±£¬ŌČÜŅŗÖŠŗ¬ÓŠĢ¼ĖįÄĘŗĶĒāŃõ»ÆÄĘŌÓÖŹ£¬¹Ź¼ÓČėµÄBČÜŅŗŹĒĻ”ŃĪĖį£¬ÄæµÄŹĒ³żČ„¹żĮæµÄĢ¼ĖįÄĘŗĶĒāŃõ»ÆÄĘ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012Äź¹ć¶«Ź”ÉĒĶ·ŹŠĮśŗžĒųÖŠæ¼Ä£Äāæ¼ŹŌ»ÆѧŹŌ¾ķ £Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
(8·Ö)ÉĒĶ·ŹĒĆĄĄöµÄŗ£±õ³ĒŹŠ£¬ŗ£ŃóŹĒ·įø»µÄ»Æѧ׏Ō“±¦æā£¬ĶعżĮĄÉ¹ŗ£Ė®æÉŅŌµĆµ½ŗ¬ÓŠ½Ļ¶ąŌÓÖŹµÄ“ÖŃĪ”£
(1)ŌŚ×ö“ÖŃĪ³õ²½Ģį“æµÄŹµŃ鏱£¬ŅŖ½ųŠŠČēĻĀĶ¼ĖłŹ¾µÄŹµŃé²Ł×÷”£
¢Ł²Ł×÷BÖŠ²£Į§°ōµÄ×÷ÓĆŹĒ
¢Ś²Ł×÷C֊擵½ Ź±£¬Ķ£Ö¹¼ÓČČ”£
(2)ÓĆĢģĘ½³ĘĮæŅ»¶ØĮæµÄŗ¬ĀČ»ÆĆ¾”¢ĮņĖįÄĘŗĶĀČ»ÆøʵēÖŃĪ£¬²¢Éč¼ĘĮĖČēĻĀĮ÷³Ģ½ųŠŠ³żŌÓĢį“棬½ųŅ»²½µĆµ½½Ļ“æ¾»µÄĀČ»ÆÄĘ¹ĢĢ唣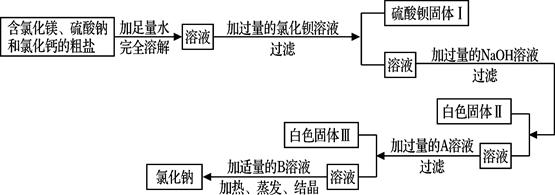
øł¾ŻĮ÷³ĢĶ¼»Ų“šĻĀĮŠĪŹĢā£ŗ
¢Ł”°¼ÓČė¹żĮæµÄAČÜŅŗ”±£¬ĘäÖŠAŹŌ¼ĮŹĒ £»
¢ŚŠ“³ö·“Ó¦Į÷³ĢÖŠ¼ÓČėĀČ»Æ±µČÜŅŗÉś³ÉÉś³ÉĮņĖį±µµÄ»Æѧ·“Ó¦·½³ĢŹ½ £»
¢Ū°×É«¹ĢĢå¢ņµÄ³É·ÖŹĒMg(OH)2£¬°×É«¹ĢĢå¢óµÄ³É·ÖŹĒ ŗĶ £»
¢Ü¼ÓČėŹŹĮæBČÜŅŗ£¬BČÜŅŗŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012ÄźÉĒĶ·ŹŠĮśŗžĒųÖŠæ¼Ä£Äāæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
(8·Ö)ÉĒĶ·ŹĒĆĄĄöµÄŗ£±õ³ĒŹŠ£¬ŗ£ŃóŹĒ·įø»µÄ»Æѧ׏Ō“±¦æā£¬ĶعżĮĄÉ¹ŗ£Ė®æÉŅŌµĆµ½ŗ¬ÓŠ½Ļ¶ąŌÓÖŹµÄ“ÖŃĪ”£
(1)ŌŚ×ö“ÖŃĪ³õ²½Ģį“æµÄŹµŃ鏱£¬ŅŖ½ųŠŠČēĻĀĶ¼ĖłŹ¾µÄŹµŃé²Ł×÷”£

¢Ł²Ł×÷BÖŠ²£Į§°ōµÄ×÷ÓĆŹĒ
¢Ś²Ł×÷C֊擵½ Ź±£¬Ķ£Ö¹¼ÓČČ”£
(2)ÓĆĢģĘ½³ĘĮæŅ»¶ØĮæµÄŗ¬ĀČ»ÆĆ¾”¢ĮņĖįÄĘŗĶĀČ»ÆøʵēÖŃĪ£¬²¢Éč¼ĘĮĖČēĻĀĮ÷³Ģ½ųŠŠ³żŌÓĢį“棬½ųŅ»²½µĆµ½½Ļ“æ¾»µÄĀČ»ÆÄĘ¹ĢĢ唣

øł¾ŻĮ÷³ĢĶ¼»Ų“šĻĀĮŠĪŹĢā£ŗ
¢Ł”°¼ÓČė¹żĮæµÄAČÜŅŗ”±£¬ĘäÖŠAŹŌ¼ĮŹĒ £»
¢ŚŠ“³ö·“Ó¦Į÷³ĢÖŠ¼ÓČėĀČ»Æ±µČÜŅŗÉś³ÉÉś³ÉĮņĖį±µµÄ»Æѧ·“Ó¦·½³ĢŹ½ £»
¢Ū°×É«¹ĢĢå¢ņµÄ³É·ÖŹĒMg(OH)2£¬°×É«¹ĢĢå¢óµÄ³É·ÖŹĒ ŗĶ £»
¢Ü¼ÓČėŹŹĮæBČÜŅŗ£¬BČÜŅŗŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com