
��4�֣�ˮ������ͨ�����������֮һ��
��1����ˮ���кܶ��֡����С�ˮ�����ڴ�������� ������ĸ��ţ���
A����ˮ B������ˮ C����Ȫˮ D������ˮ
��2���������������ˮ����Ⱦ���� (����ĸ) .
A.�������÷Ͼɵ�� B.����������Ĺ�ҵ���������ŷ�
C.��ҵ��ˮ�����������ŷ� D.�ϸ��ܻ��ʺ�ũҩ��ʹ��
��3��ˮ����Ҫ���ܼ��ͻ���ԭ�ϡ��ȼҵ�Ա���ʳ��ˮΪԭ�ϵ�����ռ�Ȼ�����Ʒ��ͬʱ�õ�һ������������壬��һ������������Ӧ�Ļ�ѧ����ʽΪ��
��1��D (2)AB
(3)2NaCl+2H2O ͨ��2NaOH+H2��+Cl2��
���������������1����Ȼ���ˮ���ǻ����������ڴ���ˮ��������ˮ��ѡD
��2�����ˮ����Ⱦ����A���������÷Ͼɵ�غ�B������������Ĺ�ҵ���������ŷ�
��3���ռ����������ƣ���������������������Ե�ⱥ��ʳ��ˮ��Ӧ�Ļ�ѧ����ʽΪ��2NaCl+2H2Oͨ�� 2NaOH+H2��+Cl2��
���㣺��Ȼ���ˮ��ˮ����Ⱦ�����Σ���ѧ����ʽ����д


 ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(2��)�����Ĺ۵����������ʵ��
��1��������һ�ֻ���� ��
��2��ȡһ�����IJ��������ȵ���뱭ˮ���ٷ���һ�����ǣ������ڲ��������Һ��λ�����ϼǺţ�������ȫ�ܽ��Һ����ڼǺ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ճ������ũҲ�������벻��ˮ
��1������ˮ��������_________��������ˮ�е���ζ��ɫ�أ���������ˮ��Ӳˮ������ˮ������_________����մ�н϶����۵����������ˮ��ϴ�ɾ�������ˮ�м���ϴ�Ӽ�������ϴ������Ϊϴ�Ӽ�������_________�����ã��ɹ�����ʹ�õĵ�ˮ��Դ���ޣ����DZ���Ҫ��Լ��ˮ����������Ľ�ˮ��������_________����
��2�����ˮ��ʵ���У�������������������_________��������һ�����������������Ȼ�����Һ�����Ʋ���Ϊ���㡢��������ȡ����_________����װƿ����ǩ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��3�֣�2014��3��22���ǵڶ�ʮ���조����ˮ�ա���3��22��28 ���ǵڶ�ʮ�߽조�й�ˮ�ܡ������Ϲ�ȷ��2014�ꡰ����ˮ�ա������������ǡ�ˮ����Դ��Water and Energy�������ҹ�����2014�ꡰ����ˮ�ա��͡��й�ˮ�ܡ������������Ϊ����ǿ�Ӻ�����������ˮ��̬��������ijУ����С���ͬѧ��Ӧ��һ���⣬����Ӻ�ˮ���м�⡣
��1����������ӵĺ�ˮԽ�����ǡ���ȥˮ�����������ʽ��еIJ����� ������ĸ����
A������ B���ᾧ C������
��2��Ϊ�˱�����ӵ�ˮ�ʣ����������������� (����ĸ)��
A������ũҩ�ͻ���
B������ˮ�����ж���ֲ������
C�������ŷ�������ˮ
D����ҵ��ˮ�������������ŷ�
��3�����ճ������У����ˮ��Ӳ�ȹ�����߲�ԭ������࣬�������� �ķ���������ˮ��Ӳ�Ⱥ�ɱ��ԭ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��7�֣�ˮ����Һ������������������ʮ����Ҫ�����á�
��1����ͼ�ǵ��ˮʵ��װ�á���ʵ������У��Թ�1������������ ��д��ˮ��ͨ�������·�Ӧ�Ļ�ѧ����ʽ ��
��2����Դˮ����������ˮ�Ĺ������������ʯ�ң���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ__ __��
��3��20��ʱ���Ȼ��Ƶ��ܽ��Ϊ36g����20��ʱ�Ȼ��Ʊ�����Һ�����ʺ��ܼ���������Ϊ ��
��4��Ϊ�˽���ũҵѡ�֣��ֽ�200g30%���Ȼ�����Һϡ��Ϊ10%���Ȼ�����Һ����Ҫ��ˮ������Ϊ ��
��5������ˮ��ͨ��������������ɱ������������������Ũ��ˮ������������豸�����������Ƿ�������й©��A��B��C��D��ʾ4�����ʣ�����ʾ��ͼ���±���A��B��һ�������·�Ӧ����C��D��
| ���� | A | B | C | D |  |
| ��ѧʽ | NH3 | Cl2 | N2 | | |
| ��ʾ��ͼ |  |  |  |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ�Dzⶨ�������������������ʵ��װ��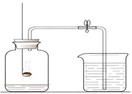
��ش������й����⣺
��1��ȼ�ճ���ʢ�ŵ������� ���ܷ�ֱ�Ӹ���ľ̿����ǵ���������ʵ�飿��˵������ ��
��2����ʵ�����ܹ۲쵽������Ϊ �����ݸ�ʵ���ԭ������ͬѧ��ΪֻҪ�Ը�ʵ������ʵ��Ľ�����ľ̿����Ǿ��ɴﵽԤ��Ŀ�ģ������жϸ�ͬѧ�����������ĸĽ��� �����ܷ����ķ�Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ˮ�ڻ�ѧʵ���е����ò��ɺ��ӡ���ͼ��ʾ���ĸ�ʵ���зֱ��õ�ˮ��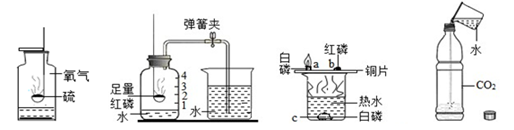
| A������������ȼ�� | B���ⶨ�������������� | C��̽��ȼ������ | D��̽��������̼���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(6��)ˮ������ͨ�����������֮һ��
��1������������Ӳˮ�����ڽ�����Ӳˮ����ˮ��������_______________________��
��2�����ˮ��֤��ˮ���⡢������Ԫ����ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��ˮ����Ҫ���ܼ��ͻ���ԭ�ϡ��ȼҵ�Ա���ʳ��ˮΪԭ�ϻ���ռ�Ȼ�����Ʒ����Ӧԭ��Ϊ:2NaCl+2H2Oͨ��2NaOH+H2��+Cl2����
�� 20��ʱ��NaCl���ܽ����36g�����¶��£�����ʳ��ˮ���������ܼ���������Ϊ ��
�� �ռ�����ڴ�������й©����Ӧ�Ļ�ѧ����ʽΪ ��
��4��ˮ�ڻ�ѧʵ���о�����Ҫ���á�����˿����
��ʪ�Ŀ����У���ͼ��ʾ����һ��ʱ���
�۲쵽������Һ������;��K���μ�ϡ���ᣬ
�۲쵽�������� ��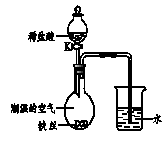
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ������������,�����ǵ�����ϢϢ��أ���ش����������еĻ�ѧ���⡣
��1������̼�����ʽ�еġ���̼��ָ����̼______(�� �����ʡ���ԭ�ӡ���Ԫ�ء�)���ճ���������ġ���̼��������� ������һ����
��2�� �豭�ڵ�ɴ���ɽ���Ҷ���ˮ����������ã������ԭ�����ƻ�ѧʵ������е�___ _���ڼ����ݲ�ʱ��Ҫ��֪��������ˮ��Ӳˮ������ˮ�ķ����� ��
��3�����ʵĽṹ�������ʵ����ʺ���;���ڼ�ͥú¯ȼ��úʱ���ѳɷ���ú����������ԭ���� ���ù��ķ�����ú��ŵ���ˮ����,������ȥ����ζ��ɫ�ص�Ч����������ú��������뾻ˮ���� __����(���й����ʼ��������)��Ϊ�˼���Դ�������Ⱦ���ö��ͥ�����˸�Ϊ��������ȼ�ϡ�����Ȼ������д����Ȼ����Ҫ�ɷ�ȼ�յĻ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com