

���� ��1��A�����ݽ���ʯ���飬������Ӧ��ĽӴ�������з�����
B�����ݴ������ص���з�����
C�����ݶ��������ˮ�����������ᣬ���������ˮ������������з�����
D�����ݿ����к������������з�����
��2�����ݻ��ϼ۴�����Ϊ����з�����
��3�����ݶ�����������������������¼�����������������з�����
��4������ϡ��Ũ�������ȷ�������з�����
��� �⣺��1��A������ʯ���飬������Ӧ��ĽӴ��������A��ȷ��
B��ʹ�ô����ܸı䷴Ӧ�����ʵȣ��������SO3�IJ�������B����
C�����������ˮ�����������ᣬ���������ˮ���������ᣬ���ܻ��������꣬��C��ȷ��
D���ų��Ŀ����к������������ɹ���������D��ȷ��
��ѡ��B��
��2��FeS2����Ϊ������������Ԫ����+2�ۣ�����Ԫ�صĻ��ϼ�Ϊx��+2+2x=0��x=-1����Ԫ��Ϊ-1�ۣ����������У�����ʾ+4�ۣ�������������Ԫ����ʾ+6�ۣ�����������Ԫ�صĻ��ϼ����Ϊ+6�ۣ�
��3��������������������������¼���������������ѧ����ʽΪ��2SO2+O2$\frac{\underline{����}}{��}$2SO3��
��4��ϡ��Ũ����ʱ��һ��Ҫ��Ũ��������������ע��ˮ�У������Ͻ��裮
�ʴ�Ϊ����1��B��
��2��+6��
��3��2SO2+O2$\frac{\underline{����}}{��}$2SO3��
��4��һ��Ҫ��Ũ��������������ע��ˮ�У������Ͻ��裮
���� �����Ĺؼ���֪����Ӧ��Ӵ����Խ��ӦԽ���ң���Ϥ����ʽ����дע�����֪��Ũ�����ϡ�ͷ�����֪ʶ��


 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | +5 | B�� | +3 | C�� | +1 | D�� | -3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
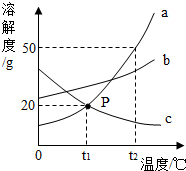 ����ͼ��a��b��c�������ʵ��ܽ�����ߣ��ش��������⣺
����ͼ��a��b��c�������ʵ��ܽ�����ߣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���ʣ����ʣ� | ѡ���Լ������� |
| A | CaCO3��CaCl2�� | �����ᣬ�������ܽ�������ᾧ |
| B | KCl��Һ��MgCl2�� | ������NaOH��Һ������ |
| C | CO2���壨ˮ������ | ������ͨ��װ������CaO�IJ����� |
| D | Cu�ۣ�Fe�ۣ� | �ӹ���ϡ���ᣬ��ַ�Ӧ����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ᄀ�������ϴ������ò��� | B�� | ʹ�ý��ܵ��ݣ��������ߵ��� | ||
| C�� | �ٿ�˽�ҳ�����˹����� | D�� | ��Լ��ˮ���ؽ�ˮ��ͷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �����д����л�ѧ��
�����д����л�ѧ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com