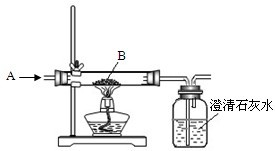
ĶŗĶĢśŹĒÉś²ś”¢Éś»īÖŠ¹ć·ŗŹ¹ÓĆµÄ½šŹō£®
£Ø1£©ÓĆŃĪĖįæÉŅŌ³żČ„ĢśŠā£ØÖ÷ŅŖ³É·ÖŹĒŃõ»ÆĢś£©£¬ĖüµÄ»Æѧ·½³ĢŹ½ŹĒ______£®
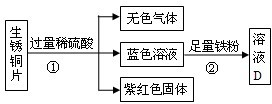
£Ø2£©ÓŠĮ½ÖÖĶŗĻ½š£Ø¢ń£©Cu-Zn£¬£Ø¢ņ£©Cu-Ag£¬½«Į½ÖÖĶŗĻ½š·Ö±š·ÅČėĻ”ĮņĖįČÜŅŗÖŠ£¬Äܹ»ÓėĻ”ĮņĖįČÜŅŗ·¢ÉśµÄĶŗĻ½šŹĒ______£ØĢī¢ń£¬¢ņ£©£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ______£®
£Ø3£©½«Ņ»ŃĻÖŲŠāŹ“¶ų²æ·Ö±ä³ÉĶĀĢ[»ÆѧŹ½ĪŖCu2£ØOH£©2CO3]µÄĶæéŃŠÄ„³É·ŪÄ©£¬ŌŚæÕĘųÖŠ³ä·Ö×ĘÉÕ£¬Éś³ÉĪļĪŖCuO£¬Ė®ŗĶ¶žŃõ»ÆĢ¼£®·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ______£®
£Ø4£©¹¤ŅµÉĻÓĆŅ»Ńõ»ÆĢ¼ŗĶ“ÅĢśæó£ØÖ÷ŅŖ³É·ÖŹĒĖÄŃõ»ÆČżĢś£©Ņ±Į¶ÉśĢś£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ______£®Ņ±Į¶2000t ŗ¬ŌÓÖŹ3%µÄÉśĢś£¬ŠčŅŖ90%µÄ“ÅĢśæóµÄÖŹĮæŹĒ______t£®
½ā£ŗ£Ø1£©ŃĪĖįÓėĢśŠāµÄÖ÷ŅŖ³É·ÖŃõ»ÆĢś·“Ӧɜ³ÉĀČ»ÆĢśŗĶĖ®£¬»Æѧ·½³ĢŹ½ĪŖ£ŗ6HCl+Fe
2O
3=2FeCl
3+3H
2O
£Ø2£©ŠæÅÅŌŚĒāµÄĒ°Ćę£¬ÄÜÓėĮņĖį·¢Éś·“Ӧɜ³ÉÉś³ÉĮņĖįŠæŗĶĒāĘų£¬»Æѧ·½³ĢŹ½ĪŖ£ŗZn+H
2SO
4ØTZnSO
4+H
2”ü£¬¶ųĶŗĶŅų¶¼ŌŚĒāµÄŗóĆę£¬²»ÄÜÓėĖį·“Ó¦£¬¹ŹŃ”I£®
£Ø3£©¼īŹ½Ģ¼ĖįĶŌŚ¼ÓČȵÄĢõ¼žĻĀÉś³ÉŃõ»ÆĶ”¢¶žŃõ»ÆĢ¼ŗĶĖ®£¬»Æѧ·½³ĢŹ½ĪŖ£ŗCu
2£ØOH£©
2CO
3
2CuO+CO
2ӟ+H
2Oӟ
ĶŗĶŃõĘųŌŚ¼ÓČČĢõ¼žĻĀÉś³ÉŃõ»ÆĶ£¬»Æѧ·½³ĢŹ½ĪŖ£ŗ2Cu+O
2
2CuO
£Ø4£©Ņ»Ńõ»ÆĢ¼ÓėĖÄŃõ»ÆČżĢśŌŚøßĪĀĢõ¼žĻĀÉś³ÉĢśŗĶ¶žŃõ»ÆĢ¼£¬»Æѧ·½³ĢŹ½ĪŖ£ŗ4CO+Fe
3O
4
3Fe+4CO
2 Éč“ÅĢśæóµÄÖŹĮæĪŖx
4CO+Fe
3O
4
3Fe+4CO
2 232 168
x”Į90% 2000t”Į3%
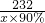
=
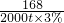
x=2977t
¹Ź“š°øĪŖ£ŗ£Ø1£©6HCl+Fe
2O
3=2FeCl
3+3H
2O
£Ø2£©I Zn+H
2SO
4ØTZnSO
4+H
2ӟ
£Ø3£©Cu
2£ØOH£©
2CO
3
2CuO+CO
2ӟ+H
2Oӟ 2Cu+O
2
2CuO
£Ø4£©4CO+Fe
3O
4
3Fe+4CO
2 2977
·ÖĪö£ŗøł¾Ż·“Ó¦Īļ”¢Éś³ÉĪļ”¢·“Ó¦Ģõ¼ž¼°ŹéŠ“»Æѧ·½³ĢŹ½µÄŌŌņŹéŠ“»Æѧ·½³ĢŹ½£®ĒāĒ°½šŹōÄÜÓėĖį·“Ó¦£¬Ēāŗó½šŹō²»ÄÜÓėĖį·“Ó¦£»øł¾Ż»Æѧ·½³ĢŹ½£¬ŅŃÖŖĢśµÄÖŹĮæĪŖ2000t”Į3%£¬æÉŅŌĒó³öĖÄŃõ»ÆČżĢśµÄÖŹĮ棬½ų¶ųĒó³ö“ÅĢśæóµÄÖŹĮ森
µćĘĄ£ŗ±¾Ģāæ¼²é»Æѧ·½³ĢŹ½µÄŹéŠ“ŅŌ¼°øł¾Ż»Æѧ·½³ĢŹ½µÄ¼ĘĖć£¬Ć÷Č·»Æѧ·½³ĢŹ½µÄŹéŠ“ŌŌņ£¬»įøł¾Ż»Æѧ·½³ĢŹ½½ųŠŠ¼ņµ„¼ĘĖćŹĒ½ā“š±¾Ģā¹Ų¼ü£®

 2CuO+CO2ӟ+H2Oӟ
2CuO+CO2ӟ+H2Oӟ 2CuO
2CuO 3Fe+4CO2
3Fe+4CO2  3Fe+4CO2
3Fe+4CO2 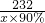 =
=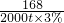
 2CuO+CO2ӟ+H2Oӟ 2Cu+O2
2CuO+CO2ӟ+H2Oӟ 2Cu+O2 2CuO
2CuO 3Fe+4CO2 2977
3Fe+4CO2 2977