
2015�����Σ��Ʒ��ը�¹ʾ�ʾ���ǣ����ʵ������ȡ�����Ч���Ĺؼ���
��1�������͡����������ʧ��ѡ�ã�_____������ͼ�顢�����������豸�����������������ʧ��ѡ�ã�_____������ľ�ġ����������ʧ��ѡ�ã�______��
A.ˮ��������� B.������̼����� C.�ɷ��������
��2������þ�Ż�����CO2�����Ϊþ����CO2��ȼ������̼������þ���÷�Ӧ�Ļ�ѧ����ʽΪ_________________________���������Ż�����ˮ�����Ϊ������ˮ��Ӧ�����������ƺ��������÷�Ӧ�Ļ�ѧ����ʽΪ__________________________��
 ������������ϵ�д�
������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ���꼶3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ�������
����ȱ�ٱ������Ԫ�ػ�Ӱ�콡����ƶѪͨ����Ҫ�����Ԫ���ǣ� ��
A. �� B. �� C. �� D. ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�챱����ʯ��ɽ�����꼶��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ�������
ͭ�ܱ��ӹ��ɺ�Ƚ�Ϊ7 ��m�ij���ͭ����˵��ͭ�������õ�
A. ��չ��
B. ������
C. ������
D. �ȶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ�����о��꼶3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ�������
���л����У����ڸ��Ϸʵ��ǣ� ��
A. NH4Cl B. NH4NO3 C. KNO3 D. Ca3(PO4)2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��㶫ʡ��ɽ��˳�������꼶��һ��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�̽����
����������мг���������ʡ�ԣ��������ͼ�ش����⣺
��1��ͼ�������ۢݵ����ƣ���________����__________��
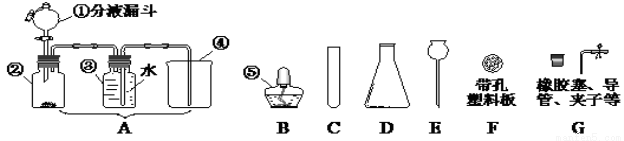
��2��ʵ������ȡ������ijͬѧ�����ͼA��װ�ã������������ռ����������У���������ʢ�ŵ��Լ�ӦΪ_______���˷����������Ļ�ѧ����ʽΪ______________________���ô˷����ռ����������϶�ˮ��������Ҫ�ռ��������������_________________�����ռ���
��3��ʵ������ȡ������̼����B~G��ѡ����������װ����װ�ã�Ҫ���ܿ��Ʒ�Ӧ�ķ�����ֹͣ������ѡC�⣬��Ҫѡ_______������ĸ������Ӧ�Ļ�ѧ����ʽΪ___________________����Ӧ����Ϊ��____________��
��4����ͼ��������ԭ����ͭ����ʵ��װ��ͼ��
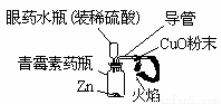
���м��ȴ�������Ϊ_______________________������ͼA��ȣ���ҩˮƿ�������൱����������(�����)_____��������������ʵ�飬���Լ������������⣬�����ܾ��е��ŵ���______________________��д1�㣩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��㶫ʡ��ɽ��˳�������꼶��һ��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ˮ��Һ���ܴ������棬����ҺΪ��ɫ����һ�������ǣ� ��
A��CaCl2��Na2CO3��KNO3 B��NaNO3��FeCl3��HCl
C��NaCl��Ba��NO3��2��NaOH D��CuSO4��KCl��HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��㶫ʡ��ɽ��˳�������꼶��һ��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�ѡ�������
ѧϰ�˻�ѧʹ���Ƕ���Ʒ��ǩ�ͱ�ʶ���˸����ε���ʶ��������ö��ʶʹ�ò�ǡ������
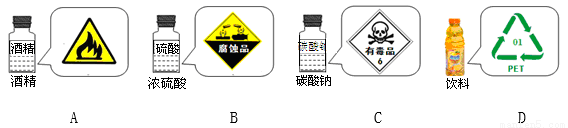
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017������ʡ���꼶��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ȷ��ȡ15mlҺ�壬Ӧѡ�õ�һ��������( )
��5mL��Ͳ ��10mL��Ͳ ��20mL��Ͳ �ܽ�ͷ�ι�
A���٢� B���ۢ� C���� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��㶫ʡ��ݸ�����dz�ѧУ���꼶��һ��ģ�⻯ѧ�Ծ��������棩 ���ͣ������
ʵ�������ⶨһƿ��ǩ�����ϡH2SO4������������������ȡ10gϡ������Ʒ����5%��NaOH��Һ��εμӵ���Ʒ�У��ӱ߽��裬����NaOH��Һ�IJ��ϼ��룬��ҺpH�ı仯����ͼ��ʾ���Իش�
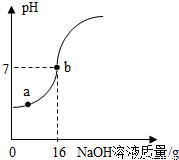
��1��a����Һ�к��е�������___________________________;
��2��;��b���ʾ�ĺ����ǣ�___________________________����ʱ����NaOH��Һ�е�NaOH������Ϊ_________g;
��3������ϡH2SO4��������������_____________����д��������̣������ȷ��0.1%����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com