
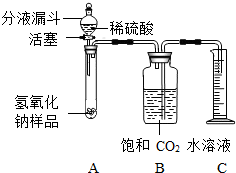
 ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ��������������������ͼ��ʾ��װ�ã�ͼ������̨����ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£�
ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ��������������������ͼ��ʾ��װ�ã�ͼ������̨����ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£�| 106 |
| x |
| 44 |
| 0.396g |
| 0.954g |
| 2g |


 ��������������������ϵ�д�
��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
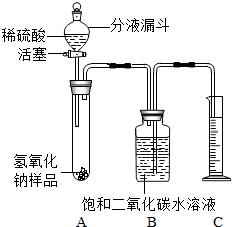 ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ�ã�ͼ������̨����ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£�
ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ�ã�ͼ������̨����ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
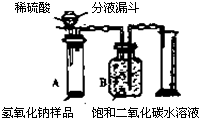 ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ�ã�ͼ������̨�Ѿ���ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£�
ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ�ã�ͼ������̨�Ѿ���ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£�| ʵ�鲽�� | ʵ������ | ʵ����� |
| ����һ�� ȡ��������ˮ���μ� �������Ȼ�����Һ ȡ��������ˮ���μ� �������Ȼ�����Һ |
�а�ɫ�������� �а�ɫ�������� |
�������Ʋ��ֱ��� |
| ������� ���ã�������Һ�еμ� ��̪ ���ã�������Һ�еμ� ��̪ |
��� ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2006�꽭��ʡ̩����̩������˼��ѧ���꼶��ѧʵ����̽��ר����ϰ�Ծ���4�·ݣ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com