
 £®ĻÖČ”øĆ³§·ĻĘųѳʷ1000L£¬ÓĆ0.0254%µÄµā£ØI2£©ČÜŅŗ2gøÕŗĆÓėÖ®ĶźČ«·“Ó¦£®ŌņøĆ³§ÅŷŵķĻĘųÖŠSO2µÄŗ¬Įæ______£ØĢī”°·ūŗĻ”±»ņ”°²»·ūŗĻ”±£©¹ś¼Ņ±ź×¼£®
£®ĻÖČ”øĆ³§·ĻĘųѳʷ1000L£¬ÓĆ0.0254%µÄµā£ØI2£©ČÜŅŗ2gøÕŗĆÓėÖ®ĶźČ«·“Ó¦£®ŌņøĆ³§ÅŷŵķĻĘųÖŠSO2µÄŗ¬Įæ______£ØĢī”°·ūŗĻ”±»ņ”°²»·ūŗĻ”±£©¹ś¼Ņ±ź×¼£® ”Į100%=50%£¬ĖłŅŌøĆ³§ĆæĢģ²śÉś¶žŃõ»ÆĮņµÄÖŹĮæĪŖ£ŗ100t”Į1.6%”Ā50%=3.2t£®
”Į100%=50%£¬ĖłŅŌøĆ³§ĆæĢģ²śÉś¶žŃõ»ÆĮņµÄÖŹĮæĪŖ£ŗ100t”Į1.6%”Ā50%=3.2t£®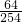 =
=



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗĶ¬²½Ģā ĢāŠĶ£ŗĢīæÕĢā
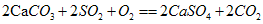 £¬ŹµŃéÖ¤Ć÷ŹÆ»Ņ½¬[Ca(OH)2]ŌŚæÕĘųÖŠŅ²æÉĪüŹÕSO2Éś³ÉĮņĖįøĘŗĶĘäĖūĪļÖŹ£¬Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ________________________________”£
£¬ŹµŃéÖ¤Ć÷ŹÆ»Ņ½¬[Ca(OH)2]ŌŚæÕĘųÖŠŅ²æÉĪüŹÕSO2Éś³ÉĮņĖįøĘŗĶĘäĖūĪļÖŹ£¬Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ________________________________”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2003ÄźµŚŹ®Čż½ģ”°ĢģŌ±”±Č«¹ś³õ֊ѧɜ»ÆѧĖŲÖŹŗĶŹµŃéÄÜĮ¦¾ŗČü£ØĮÉÄžČüĒų£©³õČüŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ”¶7.3 Ź¹ÓĆČ¼ĮĻ¶Ō»·¾³µÄÓ°Ļģ”·2010ÄźĶ¬²½Į·Ļ°£Ø1£©£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com