
����Ŀ��ijƷ�ƴ����к�������NaCl����ѧ��ȤС���ͬѧ����������ʵ��̽������ȡ12g��Ʒ�����ձ��У�����ϡ���������ٲ�������Ϊֹ�������Ƴ�����ϡ�����������ų����������Ĺ�ϵ��ͼ,
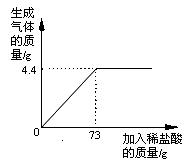
��1������ǡ����ȫ��Ӧʱ������CO2������Ϊ g
��2���������Ʒ�к����ʵ����������Ƕ��٣�������������һλС������ͬ��
��3�����㵱�����봿��ǡ����ȫ��Ӧʱ��������Һ���������������Ƕ��٣�
���𰸡���1��4.4g��2��11.7% ��3��16.3%
��������
�����������1��������Ʒ�е���Ҫ�ɷ�Ϊ̼���ƣ��������ᷴӦ���ɶ�����̼���塣����ͼʾ��֪��������73gϡ����ʱ����������������ﵽ�����ֵ������ʱ̼����ǡ����ȫ��Ӧ������ǡ����ȫ��Ӧʱ���ɶ�����̼������Ϊ4.4g��
��2�����������֪����֪��Ϊ������̼��������δ֪��Ϊ��Ʒ�к����ʵ���������������˼·Ϊ���ɸ��ݷ�Ӧ�ж�����̼��̼���Ƶ�������ϵ���̼���Ƶ���������һ���������Ʒ�к��Ȼ��Ƶ�����������
��3�����������֪����֪��Ϊ������̼��������δ֪��Ϊ������Һ�����ʵ���������������˼·Ϊ��������ҺΪ�Ȼ�����Һ���ɸ��ݷ�Ӧ�ж�����̼���Ȼ��Ƶ�������ϵ��������Ȼ��Ƶ��������ټ�����Ʒ��ԭ���Ȼ��Ƶ���������Ϊ������Һ�е����ʵ��������ٸ��������غ㶨�ɿ����������Һ�����������ɼ����������Һ�����ʵ���������������������£�
��2���⣺����Ʒ�к�̼���Ƶ�����Ϊx����Ӧ�����Ȼ��Ƶ�����Ϊy
Na2CO3+2HCl==2NaCl+H2O+CO2��
106 117 44
x y 4.4g
106��44=x��4.4g
x=10.6g
117��44=y��4.4g
y=11.7g
��Ʒ�к����ʵ���������Ϊ��![]() ��100%��11.7%
��100%��11.7%
��3��������Һ�����ʵ���������Ϊ��![]() ��100%��16.3%
��100%��16.3%
�𣺣�1�����ɶ�����̼������Ϊ4.4g��
��2����Ʒ�к����ʵ���������Ϊ11.7%��
��3��������Һ�����ʵ���������Ϊ16.3%��


 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ճ������г��������������������ڻ�ѧ�仯����( )
A��������糿���������Ͻ���һ����ı�
B�����г���̥�������±���
C���˵�����
D���õ��Ⱥ��տ�ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧϰС��������ͼ������װ�ý��������Ʊ����ش��й����⣺
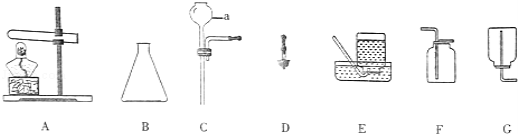
��1��д������a������ ����Aװ����ȡ�����Ļ�ѧ��Ӧ����ʽΪ ��
��2����Ҫ��ʵ������ȡ������̼���壬�䷢��װ�ÿ�ѡ��ͼ�е� ������ţ�������װ�����Ӳ����ܺͽ�Ƥ�ܵIJ������ȰѲ����ܿ� ��Ȼ�������������ɰѲ����ܲ��뽺Ƥ���ڣ�
��3��Ҫ�Ƶĸ���Ķ�����̼�������ռ�װ��ǰ����ʢ�� ��ϴ��ƿ��
��4��ʵ�����ռ������Ͷ�����̼�����õ���װ���� ������ţ�
��5���ó���ʯ��ˮ���������̼���䷴Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��֤�����ҡ������������Ļ��˳�����ֽ����ֱ����ϡ�����У�ֻ���ұ��������Ա仯���Ѽ��������������Һ�У��ı����б���������ס��ҡ������ֽ����Ļ��˳����ǿ��������
A.�ף������� B.�ң��ף��� C.�ף��ң��� D.�����ң���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����̼���80%��ʯ��ʯ125g �������м�������ϡ����ǡ����ȫ��Ӧ�������̼�������������ʶ�������ˮ���Ҳ���ϡ���ᷴӦ����
�Ŷ�����̼����
�ƶ�����̼���Ϊ������������״̬�¶�����̼���ܶ�ԼΪ2.0��/����������һλС����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ڹ����������У�Ϊ��ֹ�¹ʷ���������һЩ��ȫ��ʩ�����а�ȫ��ʩ����ȷ����
A.��ú������ú�û������ B.������úȡůʱ����Ŵ�
C.�����Ż��ù��Ǹ��� D.������ú��й©��������ͨ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ȤС���ͬѧΪ�˲ⶨ̼������Ʒ��ֻ�����Ȼ��ƣ������������ʣ���̼���Ƶĺ�����ȡ�û������Ʒ������ϡ����ǡ����ȫ��Ӧ���й�ʵ�����ݼ�������
������ʵ������
��Ӧǰ | ��Ӧ�� | |||
ʵ������ | �ձ�������/g | ϡ���������/g | �������Ʒ������/g | �ձ������л���������/g |
40.6 | 123.4 | 12 | 171.6 | |
��1����Ӧ���ɶ�����̼������Ϊ g��
��2����Ʒ̼���Ƶ����������Ƕ��٣��������������0.1%��
��3����Ӧ��������Һ���������������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭ��ͭ��п�ĺϽ�ij��ѧ��ȤС���ͬѧ�ڲⶨ��ͭ��ͭ�ĺ���ʱ��ȡ��ͭ��Ʒ40g�������ձ��У������м���200gϡ���ᣬǡ����ȫ��Ӧ����Ӧ���ձ���ʣ�����������Ϊ239.6g�������
������������������ ��
�����뷴Ӧ��ϡ����������� ��
����ͭ��ͭ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��PM2.5��ָ����ο����������������������������ⷶΧ����
A.CO2 B.SO2 C.NO2 D.PM2.5
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com