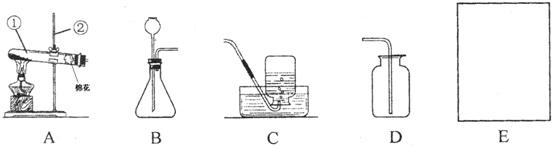
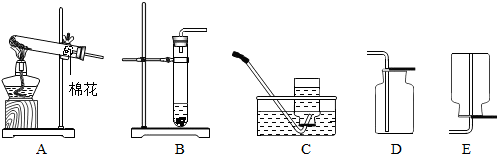
��������ʵ��װ��ͼ����ͼ1�����ش����⣮
��1��Aװ���У�����a��������
�ƾ���
�ƾ���
��
��2����Aװ�ÿ����Ʊ���һ��������
����
����
����ȡ������Ļ�ѧ����ʽ��
��ѡ��÷���װ�õ�ԭ����
��Ӧ���ǹ��壬��Ҫ����
��Ӧ���ǹ��壬��Ҫ����
�����װ�������Եķ�����
�Ȱѵ��ܵ�һ�˲���ˮ�У�Ȼ�����ֽ����Թܵ���ڣ��۲쵼�ܿ��Ƿ�������ð��
�Ȱѵ��ܵ�һ�˲���ˮ�У�Ȼ�����ֽ����Թܵ���ڣ��۲쵼�ܿ��Ƿ�������ð��
��
����Eװ���ռ������壬��ԭ����
������������ˮ
������������ˮ
�������������ռ��������װ����
D��F
D��F
����ͼ����ĸ��ţ���ͬ����
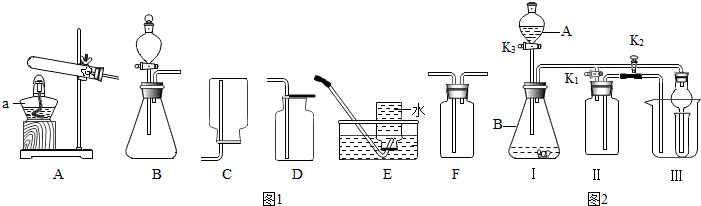
��3�������������ϣ�
����ȡNO
2�ķ�Ӧԭ����Cu+4HNO
3��Ũ���TCu��NO
3��
2+2NO
2��+2H
2O
��ͨ��״���£�NO
2��һ���ܶȱȿ�������ж����壬����ˮ������Ӧ����ȡNO
2����Ӧѡ�õķ���װ����
B
B
���ռ�װ����
D��F
D��F
��
��4����ͼ2װ�ÿ�������ȡ���壮��A��Ϊ����������Һ��B��Ϊ�������̣�����ˮ���ռ�һƿ����Ӧ���еIJ�������IIƿ��װ��ˮ��
��K1���ر�K2
��K1���ر�K2
�����ռ���һƿ������




 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�