
£Ø8·Ö£©ĻĀĶ¼ŹĒÅäÖĘČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄNaClČÜŅŗµÄŹµŃé²Ł×÷Ź¾ŅāĶ¼£ŗ
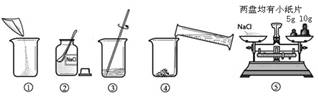

£Ø1£©ÓĆÉĻĶ¼±ķŹ¾µÄŠņŗűķŹ¾ÅäÖĘČÜŅŗµÄÕżČ·²Ł×÷Ė³Šņ______________________”£
£Ø2£©Ķ¼¢ŚÖŠ£¬ÓŠŅ»ÖÖĖÜĮĻŅĒĘ÷£¬ĘäĆū³ĘŹĒ_________”£
£Ø3£©³ĘĮæNaClŹ±£¬ĢģĘ½Ę½ŗāŗóµÄדĢ¬ČēĶ¼¢ŻĖłŹ¾£¬ÓĪĀė±ź³ßŹ¾Źż¼ūÓŅĶ¼£¬Ōņ³ĘČ”µÄNaClÖŹĮæĪŖ ”£
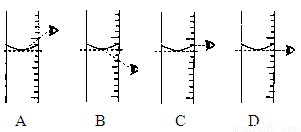
£Ø4£©øł¾Ż¼ĘĖćŠčŅŖĮæČ”Ė®µÄĢå»żŹĒ £ØĖ®µÄĆܶČĪŖ1g/mL£©”£ĮæČ”¶ĮŹżŹ±£¬ÓŅĶ¼ŹÓĻß½Ē¶ČÕżČ·µÄŹĒ_______”££ØŃ”Ģī×ÖÄø±źŗÅ£©
£Ø5£©³ĘĮæNaClÖŹĮæĶź±Ļ·Å»ŲķĄĀėŹ±£¬·¢ĻÖÓŠŅ»øöķĄĀėȱĖšĮĖŅ»øöŠ”½Ē£¬ČōĘäĖū²Ł×÷²½ÖčÕżČ·£¬ŌņĖłÅäČÜŅŗµÄČÜÖŹÖŹĮæ·ÖŹż £ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©10%”£
£Ø1£©¢Ś¢Ż¢Ł¢Ü¢Ū£Ø1·Ö£© £Ø2£©Ņ©³×£Ø1·Ö£© £Ø3£©18.2 g£Ø1·Ö£¬Ā©Š“µ„Ī»²»µĆ·Ö£© £Ø4£©163.8 ml£Ø1·Ö£¬Ā©Š“µ„Ī»²»µĆ·Ö£© D£Ø2·Ö£© £Ø5£©Š”ÓŚ£Ø2·Ö£©
”¾½āĪö”æ£Ø1£©øł¾ŻĄūÓĆ¹ĢĢåČÜÖŹĀČ»ÆÄĘÅäÖĘČÜŅŗµÄ²½Öč£¬¶Ō²Ł×÷½ųŠŠÅÅŠņ£»
£Ø2£©Ķ¼¢ŚµÄ²Ł×÷ĪŖÓĆŅ©³×Č”ÓĆ¹ĢĢå·ŪĩדĀČ»ÆÄĘ£¬ŠčŅŖŹ¹ÓƵÄŅĒĘ÷ĪŖŅ©³×£»
£Ø3£©Ź³ŃĪµÄÖŹĮæ=ķĄĀė+ÓĪĀė£¬¾ŻĶ¼æÉÖŖ£¬ķĄĀėµÄ¶ĮŹżŹĒ15g£¬ÓĪĀėµÄ¶ĮŹżŹĒ3.2g£¬¹ŹŹ³ŃĪµÄÖŹĮæ=15g+3.2g=18.2g£¬
£Ø4£©ĄūÓĆĖł³ĘČ”ČÜÖŹĀČ»ÆÄʵÄÖŹĮæŗĶ³éŅŖÅäÖĘČÜŅŗµÄČܶ“ÖŹÖŹĮæ·ÖŹż£¬øł¾ŻČÜÖŹÖŹĮæ·ÖŹż¼ĘĖć¹«Ź½£¬Ēó³öĖłŅŖÅäÖĘČÜŅŗµÄÖŹĮ棬ČÜŅŗÖŹĮæÓėČÜÖŹÖŹĮæ²ī¼“ĪŖČܼĮĖ®µÄÖŹĮ棬Ė®µÄÖŹĮæg¼“ĪŖĖ®µÄĢå»żmL£»Ź¹ÓĆĮæĶ²ĮæČ”ŅŗĢåŹ±£¬ŹÓĻßÓ¦Óė°¼ŅŗĆę×īµĶ“¦±£³ÖŌŚĶ¬Ņ»Ė®Ę½ĻßÉĻ£»
£Ø5£©ķĄĀėȱɣŅ»½ĒŌņ³ĘĮæµÄŹ³ŃĪµÄÖŹĮæ¼õŠ”£¬ČÜÖŹ¼õÉŁ£¬ŌņČÜŅŗ±äĻ”£¬ČÜÖŹÖŹĮæ·ÖŹż±äŠ”



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
6·Ö£©ĻĀĶ¼ŹĒÅäÖĘČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄNaClČÜŅŗµÄŹµŃé²Ł×÷Ź¾ŅāĶ¼£ŗ

1.ÓĆÉĻĶ¼±ķŹ¾µÄŠņŗűķŹ¾ÅäÖĘČÜŅŗµÄÕżČ·Ė³Šņ_____ ____”£
2.Ķ¼¢ŚÖŠ£¬ÓŠŅ»ÖÖĖÜĮĻŅĒĘ÷£¬ĘäĆū³ĘŹĒ___ ______”£
3.³ĘĮæNaClŹ±£¬ĢģĘ½Ę½ŗāŗóµÄדĢ¬ČēĶ¼¢ŻĖłŹ¾£¬ÓĪĀė±ź³ßŹ¾Źż¼ūÓŅĶ¼£¬Ōņ³ĘČ”µÄNaClÖŹĮæĪŖ ”£
4.øł¾Ż¼ĘĖćŠčŅŖĮæČ”Ė®µÄĢå»żŹĒ £ØĖ®µÄĆܶČĪŖ1g/mL£©”£ĮæČ”¶ĮŹżŹ±£¬ÓŅĶ¼ŹÓĻß½Ē¶ČÕżČ·µÄŹĒ_______”££ØŃ”Ģī×ÖÄø±źŗÅ£©

5.³ĘĮæNaClÖŹĮæĶź±Ļ·Å»ŲķĄĀėŹ±£¬·¢ĻÖÓŠŅ»øöķĄĀėȱĖšĮĖŅ»øöŠ”½Ē£¬ČōĘäĖū²Ł×÷²½ÖčÕżČ·£¬ŌņĖłÅäČÜŅŗµÄČÜÖŹÖŹĮæ·ÖŹż £ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©10%”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø9·Ö£©ĻĀĶ¼ŹĒÅäÖĘČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄNaClČÜŅŗµÄŹµŃé²Ł×÷Ź¾ŅāĶ¼£ŗ
£Ø1£©ÓĆÉĻĶ¼±ķŹ¾µÄŠņŗűķŹ¾ÅäÖĘČÜŅŗµÄÕżČ·²Ł×÷Ė³Šņ_______________________”£
£Ø2£©Ķ¼¢ŚÖŠ£¬ÓŠŅ»ÖÖĖÜĮĻŅĒĘ÷£¬ĘäĆū³ĘŹĒ___________”£
£Ø3£©³ĘĮæNaClŹ±£¬ĢģĘ½Ę½ŗāŗóµÄדĢ¬ČēĶ¼¢ŻĖłŹ¾£¬ÓĪĀė±ź³ßŹ¾Źż¼ūĻĀĶ¼£¬Ōņ³ĘČ”µÄNaClÖŹĮæĪŖ ”£
£Ø4£©øł¾Ż¼ĘĖćŠčŅŖĮæČ”Ė®µÄĢå»żŹĒ £ØĖ®µÄĆܶČĪŖ1g/mL£©”£ĮæČ”¶ĮŹżŹ±£¬ĻĀĶ¼ŹÓĻß½Ē¶ČÕżČ·µÄŹĒ_____”££ØŃ”Ģī×ÖÄø±źŗÅ£©
£Ø5£©³ĘĮæNaClÖŹĮæĶź±Ļ·Å»ŲķĄĀėŹ±£¬·¢ĻÖÓŠŅ»øöķĄĀėȱĖšĮĖŅ»øöŠ”½Ē£¬ČōĘäĖū²Ł×÷²½ÖčÕżČ·£¬ŌņĖłÅäČÜŅŗµÄČÜÖŹÖŹĮæ·ÖŹż £ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©10%”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012½ģĖÄ“ØŹ”³É¶¼ĢśÖŠ¾ÅÄź¼¶3ŌĀ·Ż¼ģ²ā»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø6·Ö£©ĻĀĶ¼ŹĒÅäÖĘČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄNaClČÜŅŗµÄŹµŃé²Ł×÷Ź¾ŅāĶ¼£ŗ
£Ø1£©ÓĆÉĻĶ¼±ķŹ¾µÄŠņŗűķŹ¾ÅäÖĘČÜŅŗµÄÕżČ·²Ł×÷Ė³Šņ_______________________”£
£Ø2£©Ķ¼¢ŚÖŠ£¬ÓŠŅ»ÖÖĖÜĮĻŅĒĘ÷£¬ĘäĆū³ĘŹĒ___________”£
£Ø3£©³ĘĮæNaClŹ±£¬ĢģĘ½Ę½ŗāŗóµÄדĢ¬ČēĶ¼¢ŻĖłŹ¾£¬ÓĪ Āė±ź³ßŹ¾Źż¼ūĻĀĶ¼£¬Ōņ³ĘČ”µÄNaClÖŹĮæĪŖ ”£
£Ø4£©øł¾Ż¼ĘĖćŠčŅŖĮæČ”Ė®µÄĢå»żŹĒ £ØĖ®µÄĆܶČĪŖ1g/mL£©”£ĮæČ”¶ĮŹżŹ±£¬ĻĀĶ¼ŹÓĻß½Ē¶ČÕżČ·µÄŹĒ_____”££ØŃ”Ģī×ÖÄø±źŗÅ£©
£Ø5£©³ĘĮæNaClÖŹĮæĶź±Ļ·Å»ŲķĄĀėŹ±£¬·¢ĻÖÓŠŅ»øöķĄĀėȱĖšĮĖŅ»øöŠ”½Ē£¬ČōĘäĖū²Ł×÷²½ÖčÕżČ·£¬ŌņĖłÅäČÜŅŗµÄČÜÖŹÖŹĮæ·ÖŹż £ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©10%”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012ÄźĮÉÄžŹ”ÓŖæŚŹŠÖŠæ¼Ä£Äā£Ø¶ž£©»ÆѧŹŌ¾ķ £Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
6·Ö£©ĻĀĶ¼ŹĒÅäÖĘČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄNaClČÜŅŗµÄŹµŃé²Ł×÷Ź¾ŅāĶ¼£ŗ
”¾Š”Ģā1”æÓĆÉĻĶ¼±ķŹ¾µÄŠņŗűķŹ¾ÅäÖĘČÜŅŗµÄÕżČ·Ė³Šņ_____ ____”£
”¾Š”Ģā2”æĶ¼¢ŚÖŠ£¬ÓŠŅ»ÖÖĖÜĮĻŅĒĘ÷£¬ĘäĆū³ĘŹĒ___ ______”£
”¾Š”Ģā3”æ³ĘĮæNaClŹ±£¬ĢģĘ½Ę½ŗāŗóµÄדĢ¬ČēĶ¼¢ŻĖłŹ¾£¬ÓĪĀė±ź³ßŹ¾Źż¼ūÓŅĶ¼£¬Ōņ³ĘČ”µÄNaClÖŹĮæĪŖ ”£
”¾Š”Ģā4”æøł¾Ż¼ĘĖćŠčŅŖĮæČ”Ė®µÄĢå»żŹĒ £ØĖ®µÄĆܶČĪŖ1g/mL£©”£ĮæČ”¶ĮŹżŹ±£¬ÓŅĶ¼ŹÓĻß½Ē¶ČÕżČ·µÄŹĒ_______”££ØŃ”Ģī×ÖÄø±źŗÅ£©
”¾Š”Ģā5”æ³ĘĮæNaClÖŹĮæĶź±Ļ·Å»ŲķĄĀėŹ±£¬·¢ĻÖÓŠŅ»øöķĄĀėȱĖšĮĖŅ»øöŠ”½Ē£¬ČōĘäĖū²Ł×÷²½ÖčÕżČ·£¬ŌņĖłÅäČÜŅŗµÄČÜÖŹÖŹĮæ·ÖŹż £ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©10%”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2011Äź³õÖŠ±ĻŅµÉżŃ§æ¼ŹŌ£Øɽ¶«ČÕÕÕ¾ķ£©»Æѧ ĢāŠĶ£ŗĢīæÕĢā
£Ø9·Ö£©ĻĀĶ¼ŹĒÅäÖĘČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄNaClČÜŅŗµÄŹµŃé²Ł×÷Ź¾ŅāĶ¼£ŗ

£Ø1£©ÓĆÉĻĶ¼±ķŹ¾µÄŠņŗűķŹ¾ÅäÖĘČÜŅŗµÄÕżČ·²Ł×÷Ė³Šņ_______________________”£
£Ø2£©Ķ¼¢ŚÖŠ£¬ÓŠŅ»ÖÖĖÜĮĻŅĒĘ÷£¬ĘäĆū³ĘŹĒ___________”£
£Ø3£©³ĘĮæNaClŹ±£¬ĢģĘ½Ę½ŗāŗóµÄדĢ¬ČēĶ¼¢ŻĖłŹ¾£¬ÓĪĀė±ź³ßŹ¾Źż¼ūĻĀĶ¼£¬Ōņ³ĘČ”µÄNaClÖŹĮæĪŖ ”£

£Ø4£©øł¾Ż¼ĘĖćŠčŅŖĮæČ”Ė®µÄĢå»żŹĒ £ØĖ®µÄĆܶČĪŖ1g/mL£©”£ĮæČ”¶ĮŹżŹ±£¬ĻĀĶ¼ŹÓĻß½Ē¶ČÕżČ·µÄŹĒ_____”££ØŃ”Ģī×ÖÄø±źŗÅ£©

£Ø5£©³ĘĮæNaClÖŹĮæĶź±Ļ·Å»ŲķĄĀėŹ±£¬·¢ĻÖÓŠŅ»øöķĄĀėȱĖšĮĖŅ»øöŠ”½Ē£¬ČōĘäĖū²Ł×÷²½ÖčÕżČ·£¬ŌņĖłÅäČÜŅŗµÄČÜÖŹÖŹĮæ·ÖŹż £ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©10%”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com