
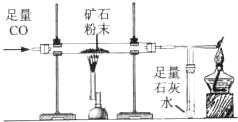
 ������ͼ��ʾװ�òⶨij��������Ŀ�ʯ��Ʒ��̼���������������������ʲ�����Ԫ������ʵ������в������κα仯����ʵ�����ݼ�¼���±��У�
������ͼ��ʾװ�òⶨij��������Ŀ�ʯ��Ʒ��̼���������������������ʲ�����Ԫ������ʵ������в������κα仯����ʵ�����ݼ�¼���±��У�| ʵ��ǰ | ʵ��� | |
| Ӳ�ʲ����ܣ�����Ʒ�� | ����185.6g | ����181.1g |
| ˵���� | �ٿ�Ӳ�ʲ���������Ϊl69.6g �ں��������ʷ�Ӧ��ȫ | |
 FeO+CO2����CO+FeO
FeO+CO2����CO+FeO Fe+CO2����CO2+Ca��OH��2�TCaCO3��+H2O��
Fe+CO2����CO2+Ca��OH��2�TCaCO3��+H2O��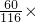 100%��=8.7g��ʵ��ǰӲ�ʲ����ܣ�����Ʒ������Ϊ185.6g����Ӳ�ʲ���������Ϊl69.6g���ó���Ʒ����Ϊ16g���ʺ�������Ŀ�ʯ��Ʒ��̼����������������Ϊ
100%��=8.7g��ʵ��ǰӲ�ʲ����ܣ�����Ʒ������Ϊ185.6g����Ӳ�ʲ���������Ϊl69.6g���ó���Ʒ����Ϊ16g���ʺ�������Ŀ�ʯ��Ʒ��̼����������������Ϊ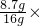 100%=54.375%��
100%=54.375%�� x=3.3g y=3.3g
x=3.3g y=3.3g


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
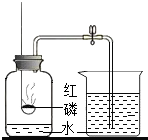 ijͬѧ������ͼ��ʾװ�òⶨ�����������ĺ��������õ��ɼм�ס��Ƥ�ܣ���ȼ���ף�����ƿ�в�����ƿ�����Ⱥ���ȼ��Ϩ����ٴ��ɼУ�
ijͬѧ������ͼ��ʾװ�òⶨ�����������ĺ��������õ��ɼм�ס��Ƥ�ܣ���ȼ���ף�����ƿ�в�����ƿ�����Ⱥ���ȼ��Ϩ����ٴ��ɼУ�| 1 | 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
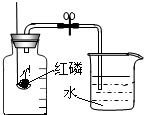 ijͬѧ������ͼ��ʾװ�òⶨ�����������ĺ��������õ��ɼм�ס��Ƥ�ܣ���ȼ���ף�����ƿ�в�����ƿ�����Ⱥ���ȼ��Ϩ����ٴ��ɼУ�
ijͬѧ������ͼ��ʾװ�òⶨ�����������ĺ��������õ��ɼм�ס��Ƥ�ܣ���ȼ���ף�����ƿ�в�����ƿ�����Ⱥ���ȼ��Ϩ����ٴ��ɼУ�| 1 | 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ij��ѧС��������ͼ��ʾװ�òⶨͭп�Ͻ���Ʒ��п������������ͼ�й̶�װ������ȥ��̽���������£�
ij��ѧС��������ͼ��ʾװ�òⶨͭп�Ͻ���Ʒ��п������������ͼ�й̶�װ������ȥ��̽���������£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
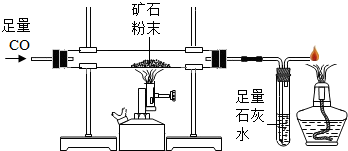 ��2013?��̨��ģ�⣩������ͼ��ʾװ�òⶨij��������Ŀ�ʯ��Ʒ��̼���������������������ʲ�����Ԫ������ʵ������в������κα仯����ʵ�����ݼ�¼���±��У�
��2013?��̨��ģ�⣩������ͼ��ʾװ�òⶨij��������Ŀ�ʯ��Ʒ��̼���������������������ʲ�����Ԫ������ʵ������в������κα仯����ʵ�����ݼ�¼���±��У�| ʵ��ǰ | ʵ��� | |
| Ӳ�ʲ����ܣ�����Ʒ�� | 185.6g | 181.1g |
| ˵ �� | �ٿ�Ӳ�ʲ���������Ϊl69.6g �ں��������ʷ�Ӧ��ȫ | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
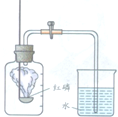 ijͬѧ������ͼ��ʾװ�òⶨ�����������ĺ��������õ��ɼм�ס��Ƥ�ܣ���ȼ���ף�����ƿ�в�����ƿ�����Ⱥ���ȼ��Ϩ����ٴ��ɼУ����ּ���ƿ�е�ˮ�������ݻ���
ijͬѧ������ͼ��ʾװ�òⶨ�����������ĺ��������õ��ɼм�ס��Ƥ�ܣ���ȼ���ף�����ƿ�в�����ƿ�����Ⱥ���ȼ��Ϩ����ٴ��ɼУ����ּ���ƿ�е�ˮ�������ݻ���| 1 |
| 5 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com