��2012?�ൺģ�⣩ij���������᳧���豸��ª�������¾ɣ��ó�ÿ���ŷŴ�����SO2�ķ����ͺ�H2SO4�����Է�ˮ�����ص����������;������ú̿��ȼ�ϣ�ͨ������ˮ�����Ƚ��вⶨ��������pHֵС��5.6����֪����ˮ�������꣬�Ըõ���������ɼ����ƻ���
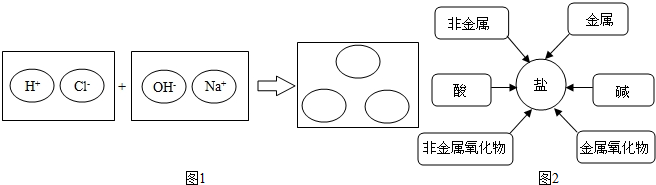
��1�����������������ԭ��
���᳧�;���ȼ��ú�ŷŵ������к�SO2���壬�������ӵı仯������ˮ�γ�����
���᳧�;���ȼ��ú�ŷŵ������к�SO2���壬�������ӵı仯������ˮ�γ�����
��2����һ��˵������Ի�����ɵ�Σ��
��ʴ����
��ʴ����
��3������ʯ�ҿ��Դ������᳧�ų������Է�ˮ������ԭ���Ļ�ѧ����ʽ��
Ca��OH��2+H2SO4�TCaSO4+2H2O
Ca��OH��2+H2SO4�TCaSO4+2H2O
��4�������ŷŵ�β����CO��SO2��NO�����ʣ�������ؿ�����Ⱦ�������������У�����������������װһ������ת����������������ʹCO��NO��Ӧ������N2��CO2��д���÷�Ӧ�Ļ�ѧ����ʽ
��



 ��У����ϵ�д�
��У����ϵ�д�