
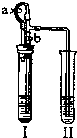 ij��ѧС��ͬѧ����ͼ��ʾװ�úͱ��������Լ�����ʵ�飨ͼ������̨�ȼг�����������ȥ����
ij��ѧС��ͬѧ����ͼ��ʾװ�úͱ��������Լ�����ʵ�飨ͼ������̨�ȼг�����������ȥ����| ��� | A�� | B�� | C�� | D�� |
| I | Ca��OH��2 ϡHCl | CaCO3 ϡHCl | Zn ϡH2SO4 | Cu ϡH2SO4 |
| �� | KNO3 | Ca��OH��2 | Ba��OH��2 | Ba��OH��2 |


 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
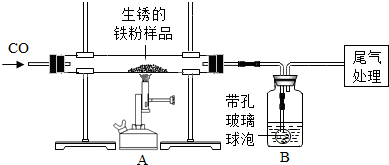
| ������ʾ�����ײ������ݿ���ʹҺ��������ֽӴ���1��װ��A�з�Ӧ�Ļ�ѧ����ʽ�� ��2��ijͬѧ��ͨ��Bװ�÷�Ӧǰ��������仯���ⶨ����Ʒ�������������������� B�е�����Լ��� �ٳ���ʯ��ˮ ����������Ũ��Һ ��ϡ���� ��ˮ ��3������ʵ������У�CO�������Ϊ��Ӧ���⣬�����������ǣ���ʵ�鿪ʼʱ���ž�װ���еĿ�������ֹ����ʱ������ը����ֹͣ���Ⱥ�ֹA�������ﱻ������B�е���Һ������A�У��� ��4����ȫ��Ӧ��ͬѧ�Ƶ����۵�����Ϊm2g��ͬʱ���װ��B����m3g����������Ʒ������������������Ϊ �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� 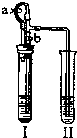 ��2007?��̨��һģ��ij��ѧС��ͬѧ����ͼ��ʾװ�úͱ��������Լ�����ʵ�飨ͼ������̨�ȼг�����������ȥ���� ��2007?��̨��һģ��ij��ѧС��ͬѧ����ͼ��ʾװ�úͱ��������Լ�����ʵ�飨ͼ������̨�ȼг�����������ȥ����
C C �飬��ʱII�з�����Ӧ�Ļ�ѧ����ʽΪBa��OH��2+H2SO4�TBaSO4��+2H2O��Ba��OH��2+ZnSO4�TBaSO4��+Zn��OH��2�� Ba��OH��2+H2SO4�TBaSO4��+2H2O��Ba��OH��2+ZnSO4�TBaSO4��+Zn��OH��2�� ���������������ԭ��������п��ϡ���ᷴӦ��������п���������ڲ���ѹ����I����Һѹ��II�У�������������Һ��Ӧ�������ᱵ���� ����п��ϡ���ᷴӦ��������п���������ڲ���ѹ����I����Һѹ��II�У�������������Һ��Ӧ�������ᱵ���� ����2����I�м����Լ���������Ƥ����������ֹˮ��a���ر�ֹˮ��b��II��������ð������Һ����ǣ���������ʵ��������Լ��� B B �飬��ʱII�з�����Ӧ�Ļ�ѧ����ʽΪCa��OH��2+CO2=CaCO3��+H2O Ca��OH��2+CO2=CaCO3��+H2O ���鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ����̨��һģ ���ͣ��ʴ��� ij��ѧС��ͬѧ����ͼ��ʾװ�úͱ��������Լ�����ʵ�飨ͼ������̨�ȼг�����������ȥ����
��2����I�м����Լ���������Ƥ����������ֹˮ��a���ر�ֹˮ��b��II��������ð������Һ����ǣ���������ʵ��������Լ���______�飬��ʱII�з�����Ӧ�Ļ�ѧ����ʽΪ______�� 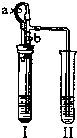 �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2007�걱���з�̨���п���ѧһģ�Ծ��������棩 ���ͣ������ ij��ѧС��ͬѧ����ͼ��ʾװ�úͱ��������Լ�����ʵ�飨ͼ������̨�ȼг�����������ȥ����
��2����I�м����Լ���������Ƥ����������ֹˮ��a���ر�ֹˮ��b��II��������ð������Һ����ǣ���������ʵ��������Լ��� �飬��ʱII�з�����Ӧ�Ļ�ѧ����ʽΪ �� 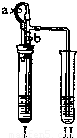 �鿴�𰸺ͽ���>> ͬ����ϰ��� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |