��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺
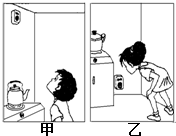
��1����Ȼ������Ϊ���ֳ��м�ͥ����Ҫ����ȼ�ϣ�Ϊ��ֹ����Ȼ��й©���Σ�գ����ڼ��а�װ���������ڼס�����ͼ�У���װλ����ȷ����
��
��
������ס����ҡ���
��2��úȼ��ʱ������
��������
��������
���γ��������Ҫ����֮һ����ˣ�ú�������ǰ�ú��Ϊ�����Դ����Ҫһ��������һ����Ҫ��Ӧ�ǣ�C+H
2O
CO+H
2���÷�Ӧ�Ļ���������
�û���Ӧ
�û���Ӧ
��
��3��ú����������ȼ�ϣ�������������ȡ�Ҷ�������ͼ����ȡ�Ҷ���������ʾ��ͼΪ��ú
��
�����Ҷ�����
�ٺϳ������л�ԭ�ԣ�д��CO��ԭ����ͭ�Ļ�ѧ����ʽ��
��
���Ҷ������Ҵ���ѧ�������ƣ��Ʋ��Ҷ�����һ����ѧ���ʣ�
���п�ȼ��
���п�ȼ��
��
�������Ժϳ�����CO��H
2��Ϊԭ�ϲ����ܵõ���������
c
c
������ĸ��ţ���
a�����ᣨHOOCCOOH����b���״���CH
3OH����c������[CO��NH
2��
2]
��4������β��ϵͳ��ʹ�ô�ת�������ɽ���CO��NO���ж�������ŷţ��䷴Ӧ��ѧ����ʽΪ��2CO+2NO
2CO
2+N
2������5.6gCO��ת��ʱ�����㣺��ͬʱ��ת����NO������������д��������̣�
6g
6g
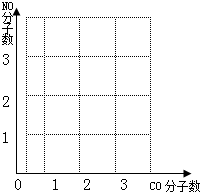
��ͼ�����к�����ΪCOΪ��������������ΪNO��������������ͼ�л��Ƴ�������Ӧ��CO��NO�仯ͼ�� �����ڴ��ֽ����ɻ�ͼ��

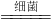 3B+5CO2+19H2O
3B+5CO2+19H2O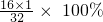 =50%��
=50%��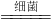 3N2+5CO2+19H2O
3N2+5CO2+19H2O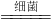 3N2+5CO2+19H2O���ɷ�Ӧ��״����������㷴Ӧ�ﰱ����������
3N2+5CO2+19H2O���ɷ�Ӧ��״����������㷴Ӧ�ﰱ����������



 ��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺
��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺