
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
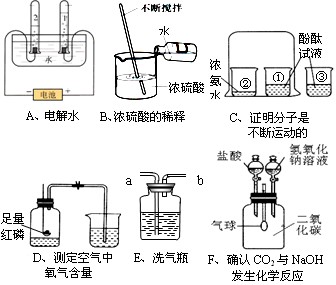
(9·Ö)ĻĀĮŠA ~ FŹĒ³õÖŠ»Æѧ֊µÄĮłøöŹµŃ飬Ēė°“ŅŖĒóĢīæÕ£ŗ

(1)CŹµŃéÖŠ½Į°čµÄÄæµÄŹĒ £®
(2)BŹµŃéÖŠŗģĮ×Č¼ÉյĻÆѧ·½³ĢŹ½ĪŖ £¬ŹµŃéĖµĆ÷ŃõĘųµÄĢå»żŌ¼Õ¼æÕĘųµÄ £¬ŹµŃé³É¹¦µÄ¹Ų¼üŹĒ (ĢīŠņŗÅ)£®
¢Ł×°ÖĆĘųĆÜŠŌŗĆ£»¢ŚŹµŃéĒ°¼Š½ōÖ¹Ė®¼Š£»¢ŪŗģĮ×¹żĮæ»ņ×ćĮ棻
¢ÜĄäČ“ŗóŌŁ“ņæŖÖ¹Ė®¼Š£»¢ŻŅŖŃ”ÓĆ½ĢŹŅÄŚµÄæÕĘų£®
(3)EŹµŃé ĻȵĪĒāŃõ»ÆÄĘČÜŅŗ£¬“ĖŹ±¹Ū²ģµ½µÄĻÖĻóŹĒ £»·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ŌŁµĪŃĪĖįŹ±¹Ū²ģµ½µÄĻÖĻóŹĒ £»·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
(4)ÉĻŹöŹµŃéÖŠÄÜ“ļµ½ŹµŃéÄæµÄĒŅÕżČ·µÄŹĒ (Ģī×ÖÄø)£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2011Äź³õÖŠ±ĻŅµÉżŃ§æ¼ŹŌ£Ø½ĖÕĖŽĒؾķ£©»Æѧ ĢāŠĶ£ŗĢīæÕĢā
£Ø14·Ö£©ĻĀĮŠA ~ FŹĒ³õÖŠ»Æѧ֊µÄĮłøöŹµŃé×°ÖĆ£¬Ēė°“ŅŖĒóĢīæÕ£ŗ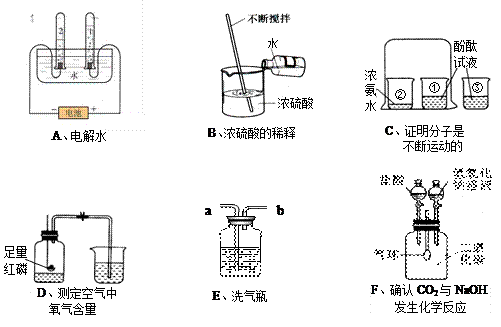
£Ø1£©AŹµŃéŹŌ¹Ü2ÖŠ²śÉśµÄĘųĢåŹĒ £¬ŹŌ¹Ü1ŗĶ2²śÉśĘųĢåµÄĢå»ż±ČŌ¼ĪŖ ”£
£Ø2£©CŹµŃéÖŠÉÕ±¢ŪµÄ×÷ÓĆŹĒ ”£
£Ø3£©DŹµŃéĖµĆ÷ŃõĘųµÄĢå»żŌ¼Õ¼æÕĘųµÄ £¬ĻĀĮŠ²Ł×÷²»Ķ׵ďĒ ”£
¢Ł¼ģ²é×°ÖĆĘųĆÜŠŌ ¢ŚŹµŃéĒ°¼Š½ōÖ¹Ė®¼Š
¢ŪĄäČ“ŗóŌŁ“ņæŖÖ¹Ė®¼Š ¢ÜŃ”ÓĆ½ĢŹŅÄŚµÄæÕĘų
£Ø4£©ČōÓĆE×°ÖĆ³żČ„O2ÖŠµÄĖ®ÕōĘų£¬øĆŅŗĢåŹŌ¼ĮĪŖ £»Ņ½Ōŗ»¹ÓĆ“Ė×°ÖĆĄ“¹Ū²ģøų²”ČĖŹäŃõĒéæö£¬µ¼¹Ü £ØĢī”°a”±»ņ”°b”±£© Ó¦Į¬½Ó²”ČĖĪüŃõĘųµÄĖܽŗ¹Ü”£
£Ø5£©FŹµŃéÖŠ£¬ĘųĒņµÄ±ä»ÆĒéæöŹĒĻČ £¬ŗó ”£Š“³öµ¼ÖĀĘųĒņ±ä»ÆµÄ»Æѧ·½³ĢŹ½ £¬ ”£
£Ø6£©ÉĻŹöŹµŃéÖŠ²»ÄÜ“ļµ½ŹµŃéÄæµÄŹĒ £ØĢī×ÖÄø£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012½ģ½ĖÕŹ”ĖÕÖŻŹŠ¾ÅÄź¼¶ÉżŃ§Ä£Äāæ¼ŹŌ»ÆѧŹŌ¾ķ £Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©ĻĀĮŠA ~ FŹĒ³õÖŠ»Æѧ֊µÄĮłøöŹµŃé×°ÖĆ£¬Ēė°“ŅŖĒóĢīæÕ£ŗ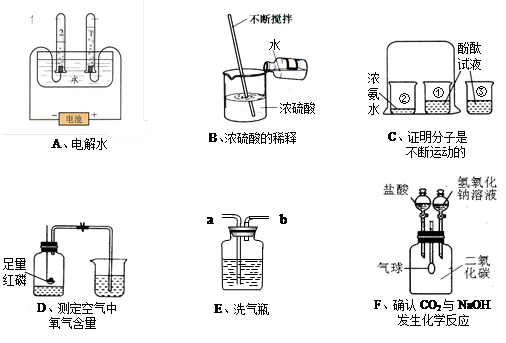
”¾Š”Ģā1”æAŹµŃéŹŌ¹Ü2ÖŠ²śÉśµÄĘųĢåŹĒ £¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
”¾Š”Ģā2”æCŹµŃéÖŠÉÕ±¢ŪµÄ×÷ÓĆŹĒ ”£
”¾Š”Ģā3”æDŹµŃéĖµĆ÷ŃõĘųµÄĢå»żŌ¼Õ¼æÕĘųµÄ £¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
”¾Š”Ģā4”æČōÓĆE×°ÖĆ³żČ„O2ÖŠµÄĖ®ÕōĘų£¬øĆŅŗĢåŹŌ¼ĮĪŖ £»
”¾Š”Ģā5”æÉĻŹöŹµŃé²Ł×÷ÖŠ²»ÕżČ·µÄŹĒ £ØĢī×ÖÄø£©”£
”¾Š”Ģā6”æFŹµŃéÖŠ£¬ĘųĒņµÄ±ä»ÆĒéæöŹĒĻČ £¬ŗó £ØĢī±ä“󔢱䊔£©”£Š“³öµ¼ÖĀĘųĒņ±ä“óµÄ»Æѧ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2009ğȫ¹śÖŠæ¼ÕęĢā×ØĢā»ć±ą×ØĢāĪåĢ½¾æĢā£ØČż£© ĢāŠĶ£ŗĢ½¾æĢā
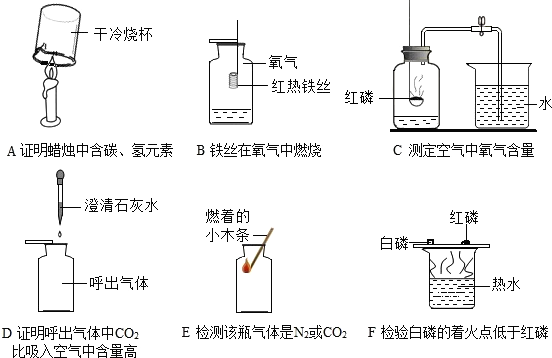
£Ø13·Ö£©ĻĀĮŠA ~ FŹĒ³õÖŠ»Æѧ֊µÄĮłøöŹµŃ飬Ēė°“ŅŖĒóĢīæÕ£ŗ
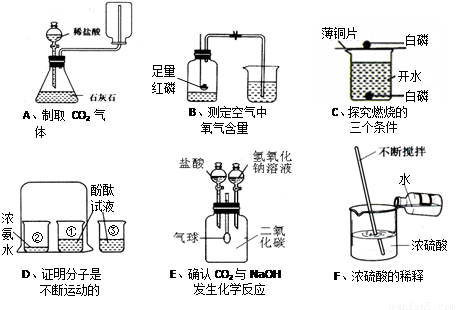
£Ø1£©DŹµŃéÖŠÉÕ±¢ŪµÄ×÷ÓĆŹĒ £¬FŹµŃéÖŠ½Į°čµÄÄæµÄŹĒ ”£
£Ø2£©BŹµŃéÖŠŗģĮ×Č¼ÉյĻÆѧ·½³ĢŹ½ĪŖ £¬ŹµŃéĖµĆ÷ŃõĘųµÄĢå»żŌ¼Õ¼æÕĘųµÄ £¬ŹµŃé³É¹¦µÄ¹Ų¼üŹĒ £ØĢīŠņŗÅ£©”£
¢Ł×°ÖĆĘųĆÜŠŌŗĆ£»¢ŚŹµŃéĒ°¼Š½ōÖ¹Ė®¼Š£»¢ŪŗģĮ×¹żĮæ»ņ×ćĮ棻¢ÜĄäČ“ŗóŌŁ“ņæŖÖ¹Ė®¼Š£»¢ŻŅŖŃ”ÓĆ½ĢŹŅÄŚµÄæÕĘų”£
£Ø3£©EŹµŃéµÄČ«¹ż³Ģ¹Ū²ģµ½µÄĻÖĻóŹĒ
£»·¢Éś·“Ó¦»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©ÉĻŹöŹµŃéÖŠÄÜ“ļµ½ŹµŃéÄæµÄĒŅÕżČ·µÄŹĒ £ØĢī×ÖÄø£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com