
���б����ٺ����нϳ��ĺ����ߣ�������Դʮ�ַḻ��
(1)��ˮɹ�οɻ�ô��Σ���ʵ�����д��ξ����ܽ⡢________��________���Ƶþ��Σ�
(2)ɹ�κ�õ���±ˮ�к���MgCl2��KCl��MgSO4�����ʣ���ͼ�����ǵ��ܽ������ʾ��ͼ��
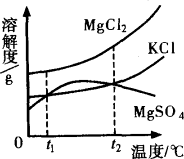
����t1��ʱMgCl2��KCl��MgSO4�������ʵ��ܽ�ȷֱ�Ϊa��b��c�������ǵĴ�С��ϵΪ________��
�ڽ�±ˮ���ȵ�t2�����ϣ������ܽ�����ߣ����������ľ�����________��
(3)Ŀǰ������60����þ�ǴӺ�ˮ����ȡ�ģ�����Ҫ�������£�

����ȡMg�Ĺ����У��Լ�A����ѡ��________���Լ�Bѡ��________������ˮMgCl2��ȡMg�ķ�Ӧ����Ϊ________��
�ڷ����Mg(OH)2���NaCl��Һ�л�����CaCl2��Na2SO4�����ʣ�Ϊ�˻��NaCl��Һ���ڷ�������Һ�����μ��������BaCl2��Һ��Na2CO3��Һ�����ˣ�������Һ�м����������ᣮʵ���м������BaCl2��Һ��Ϊ�˳�ȥ________���������Na2CO3��Һ��Ŀ����________��
(4)Ŀǰ��ˮ�����ձ���á��༶����������֤������õ���ˮΪ��ˮ�ķ�����________���������Դ����ȼ����������Ҫ�ɷ���ˮ�ϼ��龧��(CH4��nH2O)����ˮ�ϼ��龧����CH4����������Ϊ10������ˮ�ϼ��龧��Ļ�ѧʽΪ________��
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
(
��ͨ��2006)���б����ٺ����нϳ��ĺ����ߣ�������Դʮ�ַḻ��(1)
��ˮɹ�οɻ�ô��Σ���ʵ�����д��ξ����ܽ⡢________��________���Ƶþ��Σ�(2)
ɹ�κ�õ���±ˮ�к���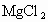 ��KCl��
��KCl��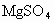 �����ʣ���ͼ�����ǵ��ܽ������ͼ��
�����ʣ���ͼ�����ǵ��ܽ������ͼ��
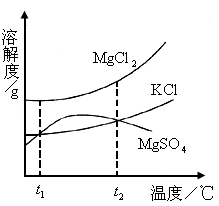
����
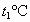 ʱ
ʱ ��KCl��
��KCl��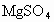 �������ʵ��ܽ�ȷֱ�Ϊa��b��c�������ǵĴ�С��ϵΪ________��
�������ʵ��ܽ�ȷֱ�Ϊa��b��c�������ǵĴ�С��ϵΪ________��
�ڽ�±ˮ���ȵ�
 ���ϣ������ܽ�����ߣ����������ľ�����________��
���ϣ������ܽ�����ߣ����������ľ�����________��
(3)
Ŀǰ��������60����þ�ǴӺ�ˮ����ȡ�ģ�����Ҫ�������£�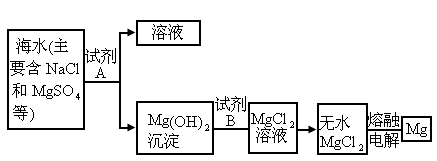
����ȡ
Mg�Ĺ����У��Լ�A����ѡ��________���Լ�Bѡ��________������ˮ ��ȡMg�ķ�Ӧ����Ϊ________��
��ȡMg�ķ�Ӧ����Ϊ________��
�ڷ����
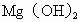 ���NaCl��Һ�л�����
���NaCl��Һ�л�����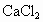 ��
��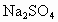 �����ʣ�Ϊ�˻��NaCl��Һ���ڷ�������Һ�����μ��������
�����ʣ�Ϊ�˻��NaCl��Һ���ڷ�������Һ�����μ��������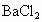 ��Һ��
��Һ��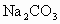 ��Һ�����ˣ�������Һ�м����������ᣮʵ���м������
��Һ�����ˣ�������Һ�м����������ᣮʵ���м������ ��Һ��Ϊ�˳�ȥ________���������
��Һ��Ϊ�˳�ȥ________���������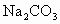 ��Һ��Ŀ����________��
��Һ��Ŀ����________��
(4)
Ŀǰ����ˮ�����ձ���á��༶����������֤������õ���ˮΪ��ˮ�ķ�����________���������Դ����ȼ����������Ҫ�ɷ���ˮ�ϼ��龧��(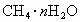 )����ˮ�ϼ��龧����
)����ˮ�ϼ��龧����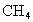 ����������Ϊ10������ˮ�ϼ��龧��Ļ�ѧʽΪ________��
����������Ϊ10������ˮ�ϼ��龧��Ļ�ѧʽΪ________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2006����ͨ�г��б�ҵ��ѧ���ԡ���ѧ ���ͣ�059
| |||||||||||||||||||
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com