�⣺��1�����������غ㣬���ձ��У�����FeSO
4������=11.2g-8g=3.2g
����ձ���CuSO
4������Ϊx
Fe+CuSO
4�TFeSO
4+Cu
160 152
x 3.2g
160��152=x��3.2g
x=3.4g
����ԭ�������CuSO
4������=3.4g��2=6.8g
��2�����������غ㣬���ձ�������������������11.2g+91.4g-100g-2.4g=0.2g
��3����Fe��H
2SO
4��Ӧ����FeSO
4������Ϊy
Fe+H
2SO
4�TFeSO
4+H
2��
152 2
y 0.2g
152��2=y��0.2g
y=15.2g
�������ձ���FeSO
4����������15.2g+3.2g=18.4g
����FeSO
4����������
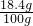
��100%=18.4%
�𣺣�1��ԭ��22.4g�����������ͭ��ĩ������6.8g��
��2�����ձ��з�Ӧ����������������0.2g��
��3�����ձ��е���������Һ��������������������18.4%��
��������1�����ձ��м�������������ˮ������������ͭ��Һ������Ӧ����ͭ���������������ڳ�ַ�Ӧ����Һ����dz��ɫ�����ж���Һ������ͭ��ȫ��Ӧ�����������غ㶨�ɣ���Ӧǰͭ�ۡ����ۺ�����ͭ��ĩ���������뷴Ӧ��ʣ�������������Ӧ�����������������������ݷ�Ӧ�Ļ�ѧ����ʽ�����������������������ɼ��㷴Ӧǰ����������ͭ����������������2����ԭ��22.4g�����������ͭ��ĩ��������
��2�������ձ��м�������ϡ���ᣬ����������۷ֱ������ἰ����ͭ������Ӧ������Ӧ����ҺΪdz��ɫ��Һ����Һ��ϡ���ἰ����ͭ��ȫ��Ӧ�����������غ㶨�ɣ����ձ��з�Ӧǰ���������������Ӧ������������������
��3�����ձ��е�������������������ͭ���������ᷴӦ���ɣ�������������ͭ��Ӧ����������������ձ�����ͬ�������ᷴӦ���ɵ����������ɸ��ݷ�Ӧ�Ļ�ѧ����ʽ�ɲ��������������㣮
���������ú������غ㶨�ɣ��DZ������һ����Ҫ�ص㣬���ձ������������غ�ɼ��㷴Ӧ�õ��������������������ձ������������غ����÷ų�����������

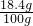 ��100%=18.4%
��100%=18.4%

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�