��ѧ������ϢϢ��أ����Ϲ���2011�궨Ϊ�����ʻ�ѧ�ꡱ���������ǡ���ѧ--���ǵ�������ǵ�δ������
��1����ѧ�뽡����Ӳˮ�����н϶ຬ�ơ�þ�����ʣ��������ò����������������________������Ӳˮ����ˮ����������ȱ�ٸ�Ԫ�ؿ��ܵ���________������ţ���
A��ƶѪ֢B����������C��٪��֢D����״�ټ���
��2����ѧ����ϣ������г����������Ŵ������Ͻ�����________���ϣ�ѡ��ϳɡ��������ϡ����������������ǽ���������
��3����ѧ����Դ����Ȼ������õĻ�ʯȼ��֮һ������Ҫ�ɷ��Ǽ��飨CH4����ȼ�յĻ�ѧ����ʽΪ________��
��4����ѧ�뻷����Ϊ���ٶ�����̼�Ի�����Ӱ�죬��ѧ�Ҽ�ǿ�˶Զ�����̼�������õ��о�����300�桢200kPa�ʹ����������£�������̼��������Ӧ����һ�����ȼ�ϼ״���CH3OH����ˮ���÷�Ӧ�Ļ�ѧ����ʽ��________��
�⣺��1����ˮ�������ˮ�����ɵ���ĭ�࣬Ӳˮ�������ˮ�����ɵ���ĭ�٣����Կ����÷���ˮ������ˮ��Ӳˮ��������ȱ�ٸ�Ԫ�ؿ��ܵ��¹������ɣ�
�ʴ�Ϊ������ˮ��B��
��2���������ϰ�����������ͭ�ȴ������ͺϽ����������г����������Ŵ������Ͻ����ڽ�����
�ʴ�Ϊ��������
��3������ȼ�����ɶ�����̼��ˮ����Ӧ����ʽ�ǣ�CH
4+2O
2
CO
2+2H
2O��
�ʴ�Ϊ��CH
4+2O
2
CO
2+2H
2O��
��4����300�桢200kPa�ʹ����������£�������̼��������Ӧ����һ�����ȼ�ϼ״���ˮ����Ӧ��ΪCO
2��H
2��������ΪCH
3OH��H
2O�������ķ�Ӧ����ʽΪ��CO
2+3H
2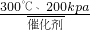
CH
3OH+H
2O��
�ʴ�Ϊ��CO
2+3H
2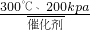
CH
3OH+H
2O��
��������1������ˮ��������������ˮ��Ӳˮ��������ȱ�ٸ�Ԫ�ؿ��ܵ��¹������ɣ�
��2���������ϰ�����������ͭ�ȴ������ͺϽ�
��3�����ݼ���ȼ�����ɶ�����̼��ˮд����ѧ����ʽ��
��4��������һ�������£�������̼��������Ӧ���ɼ״���ˮ����д��ѧ��Ӧ����ʽ��
��������ѧ��Դ����������ǵ�����ϢϢ��أ���ʵ����������ϵ�Ŀ�����ÿ���п��ıؿ���֮һ��

 CO2+2H2O��
CO2+2H2O�� CO2+2H2O��
CO2+2H2O��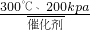 CH3OH+H2O��
CH3OH+H2O��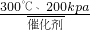 CH3OH+H2O��
CH3OH+H2O��

