
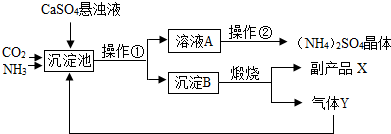
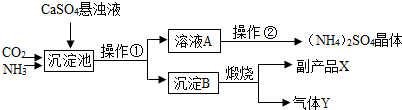
ij������Ϊ���ۺ��������������еĸ���ƷCaSO4�������ڵĻ��ʳ���������������Ʊ������[(NH4)2SO4]�Ĺ������̡�

��������ͼ�����У������������ʷ�������Ҫ��ѧ��ӦΪ��
CO2+2NH3+CaSO4+H2O =" X" ��+ (NH4)2SO4 �� ��X�Ļ�ѧʽΪ ��
�� ����B�������շ�Ӧ�Ļ�ѧ����ʽ ���ù����п�ѭ��ʹ�õ�����Ϊ ���ѧʽ����
�� �����ڵĹ����Ǽ���Ũ������ȴ���ᾧ����ã�NH4��2SO4���塣��NH4��2SO4�е��������� ���÷��ű�ʾ����
�� ����ɫ��ѧ����Դ�ۺ����õĽǶ�˵���������̵���Ҫ�ŵ���
��(��һ�㼴��)
�� CaCO3 ��
CaCO3 CaO+CO2��
CO2
CaO+CO2��
CO2
�� NH4 +
�� ������CO2ѭ��ʹ��;�õ��IJ�Ʒ����Ʒ�������õ�����;���������
��������
����������Ÿ��������غ㶨�ɣ���ѧ��Ӧǰ���ԭ�����ࡢ��Ŀ�������仯������X�Ļ�ѧʽΪCaCO3���� ����BΪ̼��ƣ������������������ƺͶ�����̼����ͼ��֪��������̼����ѭ��ʹ�ã��ǣ�NH4��2SO4�е���������笠����ӣ��������̵���Ҫ�ŵ��Dz�����CO2ѭ��ʹ��;�õ��IJ�Ʒ����Ʒ�������õ�����;���������
���㣺������ѧ��Ӧ���ƶ�
�����������Ļ�ѧ��Ӧ������Ϊ�ƶ�����زij��֣��п����о����п��죬������ÿ�궼�����⣬��ʱ��ѡ�����г��֣�������Ҫ��ƽʱҪע����ۺ��ܽᡣ



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ����С�ͤ�������꼶�ڶ��ε��в��Ի�ѧ�Ծ��������棩 ���ͣ������
ij������Ϊ���ۺ��������������еĸ���ƷCaSO4 �������ڵĻ��ʳ���������������Ʊ�(NH4)2SO4�Ĺ������̡�����ͼ�����У������������ʷ�������Ҫ��ѧ��ӦΪ��
CO2+2NH3+CaSO4+H2O = CaCO3��+(NH4)2SO4 ��
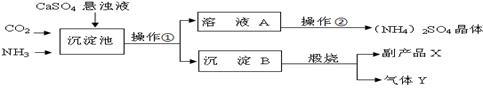
��1������B�������շ�Ӧ�Ļ�ѧ����ʽ ����Ӧ�Ļ����������� ���ù����п�ѭ��ʹ�õ�����Ϊ ���ѧʽ����
��2��ʵ����������ٳ�Ϊ ��ʵ���ҽ��д˲���ʱ���õ����������������� �������ڵĹ����Ǽ���Ũ���� ���ᾧ�����(NH4)2SO4���塣
��3������ɫ��ѧ����Դ�ۺ����õĽǶ�˵���������̵���Ҫ�ŵ���
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com