
ČĖĆĒÉś»īŌŚ”°Ė®Ēņ”±ÉĻ£¬µŲĒņ±ķĆęµÄ70.8%±»Ė®ø²øĒ”£
£Ø1£©ŗ£Ė®É¹ŃĪŹĒ½čÖśČÕ¹āŗĶ·ēĮ¦Ź¹ŗ£Ė®ÖŠµÄ_________£ØŠ“»ÆѧŹ½£©Õō·¢£¬µĆµ½ŗ¬ÓŠ½Ļ¶ąŌÓÖŹµÄ____£ØŠ“»ÆѧŹ½£©¾§Ģå.
£Ø2£©½µÓźŹĒ×ŌČ»½ēÖŠĖ®Ń»·µÄŅ»øö»·½Ś£¬____________ĘųĢå»ņÕāŠ©ĘųĢåŌŚæÕĘųÖŠ·“Ó¦ŗóµÄÉś³ÉĪļČÜÓŚÓźĖ®£¬»įŠĪ³ÉĖįÓź”£A”¢B”¢CČżøöµŲĒųÓźĖ®µÄpHČēĶ¼ĖłŹ¾£¬ĘäÖŠ_________µŲĒųµÄÓźĖ®ŹĒĖįÓź”£
£Ø3£©Ēė°“ŅŖĒ󊓳ö³õÖŠ»Æѧ½Ģ²ÄÖŠĖ®×÷ĪŖ·“Ó¦ĪļµÄ»Æѧ·½³ĢŹ½£ØĮ½øö»ÆŗĻ·“Ó¦ÖŠÉś³ÉĪļµÄĄą±š±ŲŠė²»Ķ¬£©£ŗ
¢Ł·Ö½ā·“Ó¦£ŗ_______________________________________________£»
¢Ś»ÆŗĻ·“Ó¦£ŗ_______________________________________________£»
¢Ū»ÆŗĻ·“Ó¦£ŗ_______________________________________________”£
£Ø1£©H2O£»NaCl £Ø2£©¶žŃõ»ÆĮņ£»AB £Ø3£©¢Ł2H2O 2H2”ü+O2”ü
2H2ӟ+O2ӟ
¢ŚCO2+H2O£½H2CO3 ¢ŪCaO+H2O£½Ca(OH)2
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©ŗ£Ė®É¹ŃĪŹĒ½čÖśČÕ¹āŗĶ·ēĮ¦Ź¹ŗ£Ė®ÖŠµÄĖ®Õō·¢£¬µĆµ½ŗ¬ÓŠ½Ļ¶ąŌÓÖŹµÄĀČ»ÆÄĘ¾§Ģ壬Ė®ŗĶĀČ»ÆÄʵĻÆѧŹ½·Ö±šŹĒH2O”¢NaCl”£
£Ø2£©ĖįÓźŹĒÓÉÓŚČĖĄą“óĮæŹ¹ÓĆŗ¬ĮņĮæŗÜøßµÄĆŗ”¢ŹÆÓĶµČ»ÆŹÆČ¼ĮĻ£¬Č¼ÉÕŗó²śÉśµÄ¶žŃõ»ÆĮņ”¢¶žŃõ»ÆµŖµČ£¬ŌŚ“óĘųÖŠ¾¹żø“ŌӵĻÆѧ·“Ó¦ŗóČÜÓŚÓźĖ®£¬ŠĪ³ÉĖįÓź£»¶žŃõ»ÆĮņ”¢¶žŃõ»ÆµŖŹĒŠĪ³ÉĖįÓźµÄÖ÷ŅŖĪļÖŹ”£pH£¼5.6µÄÓźĖ®ŹōÓŚĖįÓź£¬ÓÉA”¢B”¢CČżøöµŲĒųÓźĖ®µÄpHĶ¼£¬A”¢B“¦µÄÓźĖ®µÄPH£¼5.6£¬ŹōÓŚĖįÓź”£
£Ø3£©¢ŁĖ®Ķصē·Ö½āÉś³ÉĒāĘųŗĶŃõĘų£¬ŹōÓŚ·Ö½ā·“Ó¦£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ2H2O 2H2”ü+O2”ü”£
2H2ӟ+O2ӟӣ
¢Ś¶žŃõ»ÆĢ¼ŗĶĖ®·“Ӧɜ³ÉĢ¼Ėį£¬ŹōÓŚ»ÆŗĻ·“Ó¦£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖCO2+H2O£½H2CO3”£
¢ŪŃõ»ÆøĘŗĶĖ®·“Ӧɜ³ÉĒāŃõ»ÆøĘ£¬ŹōÓŚ»ÆŗĻ·“Ó¦£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖCaO+H2O£½Ca(OH)2”£
æ¼µć£ŗæ¼²éŗ£Ė®É¹ŃĪµÄŌĄķŗĶ¹ż³Ģ£»ĖįÓźµÄ²śÉś”¢Ī£ŗ¦¼°·ĄÖĪ£»ŹéŠ“»Æѧ·½³ĢŹ½”¢ĪÄ×Ö±ķ“ļŹ½


 Ķ¬²½Ń§µäŅ»æĪ¶ąĮ·ĻµĮŠ“š°ø
Ķ¬²½Ń§µäŅ»æĪ¶ąĮ·ĻµĮŠ“š°ø ¾µäĆܾķĻµĮŠ“š°ø
¾µäĆܾķĻµĮŠ“š°ø ½šÅĘæĪĢĆĮ·ĻµĮŠ“š°ø
½šÅĘæĪĢĆĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¹Ū²ģ”¢±Č½ĻÓė¹éÄÉŹĒѧĻ°»ÆѧµÄÖŲŅŖ·½·Ø”£¶ŌÓŚŅŌĻĀČżøö»Æѧ·½³ĢŹ½£ŗ
2CO + O2 2CO2 2Mg + O2
2CO2 2Mg + O2 2MgO 4P+ 5O2
2MgO 4P+ 5O2  2P2O5
2P2O5
Ķعż±Č½Ļ£¬·¢ĻÖĖüĆĒÓŠŠķ¶ą¹²Ķ¬µć£¬ĒėÄ抓³öĘäÖŠĮ½µć£ŗ
¢Ł ¢Ś
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
½«æÕĘų³ż³¾¾»»Æ£¬³żČ„¶žŃõ»ÆĢ¼ŗĶĖ®ÕōĘųŗó£¬ŌŚµĶĪĀ¼ÓŃ¹Ģõ¼žĻĀŹ¹æÕĘų_______£¬Č»ŗóæŲÖĘĪĀ¶Č·ÖĄėŅŗĢ¬æÕĘų£¬µŖĘų·Šµć±ČŃõĘų £¬ĻČÕō·¢³öĄ“µÄŹĒ £¬ÓąĻĀµÄÖ÷ŅŖŹĒ £¬øĆ¹ż³ĢĪŖ £ØĢī”°ĪļĄķ±ä»Æ”±»ņ”°»Æѧ±ä»Æ”±£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ė®ŹĒÉśĆüÖ®Ō“£¬ČĖĄąµÄČÕ³£Éś»īÓė¹¤Å©ŅµÉś²ś¶¼Ąė²»æŖĖ®”£×ŌČ»½ē“ó²æ·ÖĖ®²»ÄÜÖ±½ÓŹ¹ÓĆ£¬¶¼ŹĒŅŖ¾¹ż¾»»Æŗó·½æÉŹ¹ÓĆ”£øł¾ŻĖłŃ§ÖŖŹ¶»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©ŗéĄŌµŲĒųµÄŌÖĆń½«ŗÓĖ®×Ŗ»ÆĪŖæÉŅūÓĆĖ®µÄ²½ÖčÓŠ£ŗ
¢ŁÓĆĆ÷·Æ½ųŠŠ»Æѧ³Į½µ£» ¢ŚÓĆĘÆ°×·ŪĻū¶¾É±¾ś£» ¢Ū¹żĀĖ£» ¢Ü¼ÓČČÖó·Š”£
ÉĻŹö“¦Ąķ¹ż³ĢÖŠÓŠ¹Ų²½ÖčŗĻĄķµÄĖ³ŠņŹĒ £ØĢīŠņŗÅ£©”£
£Ø2£©Šķ¶ąµŲĻĀĖ®ŗ¬ÓŠ½Ļ¶ąµÄøĘ”¢Ć¾Ąė×Ó£¬ÕāÖÖĖ®½ŠÓ²Ė®£¬²»ÄÜÖ±½ÓŅūÓĆ”£ĒėÓĆÄćĖłŃ§ÖŖŹ¶Ą“Ö¤Ć÷ij³ĒŹŠµÄµŲĻĀĖ®ŹĒÓ²Ė®£¬·½·ØŹĒ ”£
£Ø3£©Ė®ŌŚ×ŌČ»»·¾³ÖŠ²»Ņ×·Ö½ā£¬µ«ŌŚĶصēµÄĢõ¼žĻĀæÉŅŌ·Ö½ā£¬Š“³öøĆ·“Ó¦µÄ·ūŗűķ“ļŹ½ £¬ŌŚÓŅĶ¼ĖłŹ¾µÄ×°ÖĆÖŠ£¬µ±µēŌ“½ÓĶØŅ»¶ĪŹ±¼äŗ󣬼׹ÜÖŠĘųĢåµÄĢå»żÓėŅŅ¹ÜÖŠĘųĢåµÄĢå»żÖ®±ČĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĒāÄÜŹĒ21ŹĄ¼Ķ×īĄķĻėµÄÄÜŌ“£¬µ«ÖĘĒā»¹Ć»ÓŠĻė³öÕęÕżŗĻŹŹµÄ·½·Ø£¬ÓŅĶ¼ŹĒµē½āĖ®ÖĘĒāµÄ¼ņŅ××°ÖĆĶ¼”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
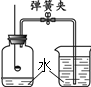
£Ø1£©µē½āĖ®ŹµŃéĖłÓƵ½µÄµēŌ“ŹĒ £ØĢī”°Ö±Į÷µē”±»ņ”°½»Į÷µē”±£©”£
£Ø2£©¼×¹ÜÉś³É____________ĘųĢ壬bÓ¦½ÓµēŌ“_____________¼«”£
£Ø3£©¼ģŃéŅŅ¹ÜÖŠĘųĢåµÄ·½·ØŹĒ ”£
£Ø4£©ĄķĀŪÉĻ¼×”¢ŅŅĮ½ŹŌ¹ÜÖŠĘųĢåµÄĢå»ż±ČĪŖ________”£
£Ø5£©ĶعżŅŌÉĻŹµŃéµĆ³öµÄ½įĀŪŹĒ___________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŃõĘųŹĒÉś²śÉś»īÖŠÖŲŅŖµÄĪļÖŹ”£
£Ø1£©ŃõĘųÓŠŗܶąÓĆĶ¾”£ĻĀĮŠŹōÓŚŃõĘųÓĆĶ¾µÄŹĒ____________£ØĢīŠņŗÅ£©”£ 
| A£®Ņ½ĮĘ¼±¾Č | B£®Ź³Ę··ĄøÆ | C£®ŗ½Ģģ»š¼ż | D£®ÄŽŗēµĘ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĪŅ¹śµÄĮģŗ£Ö÷Čز»ČŻĒÖ·ø£¬ÄĻŗ£ŹĒĪŅ¹śµÄ¹ĢÓŠĮģŗ££¬ŌĢ²Ų×Å·įø»µÄŗ£Ńó׏Ō“£®
£Ø1£©ÄĻŗ£²»½öŌĢŗ¬×Å“óĮæµÄĆŗ”¢ŹÆÓĶ”¢ĢģČ»ĘųµČ³£¹ęÄÜŌ“£¬»¹ŌĢ²Ų×Å“óĮæµÄæÉČ¼±ł£®æÉČ¼±ł£ØÖ÷ŅŖ³É·ÖŹĒCH4£©±»æĘѧ¼ŅÓžĪŖ”°Ī“Ą“ÄÜŌ“”±£¬CH4Č¼ÉյĻÆѧ·½³ĢŹ½ £¬æÉČ¼±ł×÷ĪŖÄÜŌ“ÓėĆŗ”¢ŹÆÓĶĻą±ČµÄÓŵćŹĒ £®
£Ø2£©ÄĻŗ£Ä³µŗ²ÉÓĆ·ēĮ¦·¢µēĢį¹©µÄµēÄܶŌŗ£Ė®½ųŠŠĮĖČēĶ¼1ĖłŹ¾µÄ×ŪŗĻĄūÓĆ£®
¢Ł·“ÉųĪö·Øµ»Æŗ£Ė®ŹĒĄūÓĆŗ£Ė®ÖŠø÷³É·ÖµÄ ²»Ķ¬·ÖĄė³öµĖ®£®
¢Ś½«øßŃĪ¶ČÅØĖõŗ£Ė®½ųŠŠ æÉŅŌ·ÖĄėµĆµ½“ÖŃĪ£®
¢ŪÓĆæąĀ±ÖĘČ”½šŹōĆ¾µÄĮ÷³ĢĶ¼ČēĶ¼2£ŗ
ÉĻŹö×Ŗ»Æ¹ż³ĢÖŠ£¬Ėł·¢ÉśµÄ·“Ó¦ŹōÓŚø“·Ö½ā·“Ó¦ĄąŠĶµÄ²½ÖčŹĒ £ØĢīŠņŗÅ£©£¬²½Öč¢ņµÄ»Æѧ·½³ĢŹ½ĪŖ £®ĄūÓĆæąĀ±ÖĘČ”ĒāŃõ»ÆĆ¾±Č”°Ö±½ÓĻņŗ£Ė®ÖŠ¼ÓČėŹÆ»ŅČéÖĘČ”ĒāŃõ»ÆĆ¾”±µÄÓÅŹĘŹĒ £®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÄÜŌ“”¢»·¾³”¢²ÄĮĻÓėČĖĄąµÄÉś»īĻ¢Ļ¢Ļą¹Ų£®øł¾ŻĖłŃ§ÖŖŹ¶»Ų“šĻĀĮŠĪŹĢā£®
£Ø1£©Ė®ŹĒÉśĆüÖ®Ō“£¬±£»¤Ė®×ŹŌ““ÓĪŅ×öĘš£®
¢Łµē½āĖ®ŹŌŃéÖŠ£¬ĶłĖ®ÖŠ¼ÓČėÉŁĮæĒāŃõ»ÆÄʵÄÄæµÄŹĒ”” ””£¬ŹŌŃ鏱ČōÕż¼«²śÉś6mlĘųĢ壬Ōņøŗ¼«²śÉśĘųĢåµÄĢå»żŹĒ”” ””ml£¬øĆŹµŃéÖ¤Ć÷ĮĖĖ®ŹĒÓÉ”” ””×é³ÉµÄ£®
¢ŚÉś»īÖŠ³£ÓĆ”” ””Ą“½µµĶĖ®µÄÓ²¶Č£®ČēĶ¼ĖłŹ¾ŹĒ³£ÓĆ×ŌÖĘ¾»Ė®Ę÷£¬ĘäÖŠ»īŠŌĢæµÄ×÷ÓĆŹĒ”” ””£»ÖĘŌģĖÜĮĻĘæµÄ²ÄĮĻŹōÓŚ”” ””²ÄĮĻ£®Ēė½éÉÜŅ»ÖÖÄćµÄ½ŚĖ®·½·Ø”” ””£®
£Ø2£©»ÆŹÆČ¼ĮĻŹĒÓŠĻŽµÄ£¬“óĮæŹ¹ÓĆ»įµ¼ÖĀ»·¾³ĪŪČ¾£®ĒėÄ楿¾ŁŅ»ÖÖĒå½ąÄÜŌ“”” ””£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
Č¼ĮĻµÄŹ¹ÓĆ“Ł½ųĮĖ¾¼ĆµÄ·¢Õ¹£¬øųČĖĆĒµÄÉś»ī“ųĄ“ĮĖ±ćĄū”£µ«ĆŗČ¼ÉÕŹ±£¬ÅŷŵÄĪŪČ¾Īļ»įµ¼ÖĀĖįÓźµÄ·¢Éś”£ÕāŅ»ĪŹĢāČÕŅęŹÜµ½ČĖĆĒµÄ¹Ų×¢ŗĶÖŲŹÓ£¬ĒėÄćĖµ³öĖįÓźµÄĪ£ŗ¦¼°·ĄÖĪ“ėŹ©”££ØĪ£ŗ¦“šŅ»µć£¬“ėŹ©“šĮ½µć¼“æÉ£©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com