
·ÖĪö £Ø1£©»Æѧ·“Ó¦×ńŃÖŹĮæŹŲŗć¶ØĀÉ£¬¼“²Ī¼Ó·“Ó¦µÄĪļÖŹµÄÖŹĮæÖ®ŗĶ£¬µČÓŚ·“Ó¦ŗóÉś³ÉµÄĪļÖŹµÄÖŹĮæÖ®ŗĶ£»
£Ø2£©øł¾Ż¶žŃõ»ÆĢ¼ŗĶĖ®»įÉś³ÉĢ¼Ėį½ųŠŠ·ÖĪö£»
£Ø3£©øł¾ŻĢ¼µÄ»ÆѧŠŌÖŹ·ÖĪö½ā“š£»
£Ø4£©ĢśÓėĮņĖįĶČÜŅŗ·“Ӧɜ³ÉĮņĖįŃĒĢśČÜŅŗŗĶĶ£¬Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½¼“æÉ£»
£Ø5£©øł¾Ż¶žŃõ»ÆĮņµÄŠŌÖŹ·ÖĪö£®
½ā“š ½ā£ŗ£Ø1£©Ć¾ĢõŌŚæÕĘųÖŠČ¼ÉÕŹ±£¬Ć¾ŗĶæÕĘųÖŠµÄŃõĘų·“Ӧɜ³ÉŃõ»ÆĆ¾£¬Éś³ÉµÄŃõ»ÆĆ¾µÄÖŹĮæµČÓŚ²Ī¼Ó·“Ó¦µÄĆ¾µÄÖŹĮæŗĶŃõĘųµÄÖŹĮæÖ®ŗĶ£¬Ņņ“ĖÉś³ÉĪļµÄÖŹĮæ±ČŌĄ“Ć¾ĢõµÄÖŹĮæŌö“ó£»
£Ø2£©¶žŃõ»ÆĢ¼ŗĶÓėĖ®·“Ӧɜ³ÉĢ¼Ėį£¬Ģ¼Ėį¾ßÓŠČõĖįŠŌ£»
£Ø3£©Ä¾ĢõµÄÖ÷ŅŖ³É·ÖĪŖĢ¼£¬¹ŹĢ¼ÓėŃõĘųµćČ¼µÄĢõ¼žĻĀÉś³É¶žŃõ»ÆĢ¼£»
£Ø4£©ĢśÓėĮņĖįĶČÜŅŗ·“Ӧɜ³ÉĮņĖįŃĒĢśČÜŅŗŗĶĶ£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗFe+CuSO4ØTFeSO4+Cu£®
£Ø5£©ŅņĪŖĮņČ¼ÉÕÉś³É¶žŃõ»ÆĮņ£¬¶žŃõ»ÆĮņÓŠ¶¾µ«ÄÜÓėĖ®·“Ӧɜ³ÉŃĒĮņĖį£»
“š°ø£ŗ£Ø1£©ŅņĪŖÉś³É¹ĢĢåµÄÖŹĮæµČÓŚ²Ī¼Ó·“Ó¦µÄ¹ĢĢåĆ¾Óė²Ī¼Ó·“Ó¦µÄŃõĘųµÄÖŹĮæŗĶ£®
£Ø2£©CO2+H2O=H2CO3£»
£Ø3£©C+O2 $\frac{\underline{\;µćČ¼\;}}{\;}$CO2£»
£Ø4£©Fe+CuSO4=FeSO4+Cu£»
£Ø5£©SO2+H2O=H2SO3£®
µćĘĄ ±¾ĢāÄŃ¶Č²»“ó£¬ÕĘĪÕĄūÓĆ·Ö×ӵĻł±¾ŠŌÖŹ·ÖĪöŗĶ½ā¾öĪŹĢāµÄ·½·Ø”¢»Æѧ·½³ĢŹ½µÄŹéŠ“·½·Ø£Ø¾³£³öĻֵēķĪóÓŠ²»·ūŗĻæĶ¹ŪŹĀŹµ”¢²»×ńŹŲÖŹĮæŹŲŗć¶ØĀÉ”¢²»Š“Ģõ¼ž”¢²»±ź·ūŗÅµČ£©ŹĒ½ā“š“ĖĄąĢāµÄ¹Ų¼ü£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  | B£® |  | C£® |  | D£® |  |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
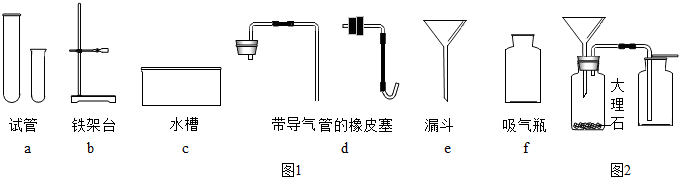
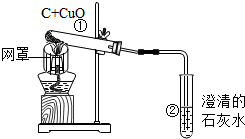 ÓĆľĢ滹ŌŃõ»ÆĶµÄŹµŃéČēĶ¼£®
ÓĆľĢ滹ŌŃõ»ÆĶµÄŹµŃéČēĶ¼£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  ³ĘČ”Ź³ŃĪ | B£® |  Ļ”ŹĶÅØĮņĖį | C£® |  ²ā¶ØČÜŅŗµÄpH | D£® |  ¹ĢĢåŅ©Ę·µÄČ”ÓĆ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ²Ī¼Ó·“Ó¦µÄO2µÄÖŹĮæŹĒ11.2g | B£® | øĆĪļÖŹÖŠĢ¼”¢ĒāŌŖĖŲÖŹĮæ±ČŹĒ1£ŗ3 | ||
| C£® | Éś³ÉCO2ŗĶH2OµÄ·Ö×ÓøöŹż±ČĪŖ2£ŗ3 | D£® | øĆĪļÖŹÖŠ²»ŗ¬ŃõŌŖĖŲ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ńõ»ÆĪļ | B£® | Ėį | C£® | ¼ī | D£® | ŃĪ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com