
 £»x=5.4g£¬ŌņŃõ»ÆĀĮµÄÖŹĮæŹĒ15.6g-5.4g=10.2g
£»x=5.4g£¬ŌņŃõ»ÆĀĮµÄÖŹĮæŹĒ15.6g-5.4g=10.2g £¬y=21.9g
£¬y=21.9g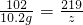 £¬Ōņz=21.9g£¬ÓÉÓė·“Ó¦ŗóČÜŅŗÖŠÖ»ŗ¬ÓŠŅ»ÖÖČÜÖŹ£¬Ōņ50gŃĪĖįŅ²ŹĒĒ”ŗĆ·“Ó¦£¬Ōņ
£¬Ōņz=21.9g£¬ÓÉÓė·“Ó¦ŗóČÜŅŗÖŠÖ»ŗ¬ÓŠŅ»ÖÖČÜÖŹ£¬Ōņ50gŃĪĖįŅ²ŹĒĒ”ŗĆ·“Ó¦£¬Ōņ =87.6%
=87.6% =£Ø21.9g+21.9g£©”Į
=£Ø21.9g+21.9g£©”Į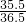 £¬w=53.4g£¬ĖłŅŌĻņ·“Ó¦ŗóČÜŅŗÖŠ¼ÓČė35gĖ®£¬¼ÓĖ®ŗóČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ
£¬w=53.4g£¬ĖłŅŌĻņ·“Ó¦ŗóČÜŅŗÖŠ¼ÓČė35gĖ®£¬¼ÓĖ®ŗóČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ ”Į100%=53.4%£»
”Į100%=53.4%£» £»£Ø3£©87.6%£»£Ø4£©53.4%£»£Ø5£©25£»
£»£Ø3£©87.6%£»£Ø4£©53.4%£»£Ø5£©25£»

 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| 54 |
| x |
| 6 |
| 0.6g |
| 54 |
| x |
| 6 |
| 0.6g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2013Äź5ŌĀÖŠæ¼»ÆŃ§Ä£ÄāŹŌ¾ķ£Ø10£©£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com