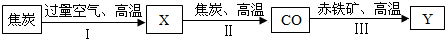��2012?������һģ����ѧ����������������أ�
��1�������е�������Ʒ����ʹ�õ���Ҫ����������Ȼ��ά����
C
C
������ĸ��ţ���

��2������ʳ���У����ṩ����ά���ص���
A
A
������ĸ��ţ�

��3���ӵ����С��⡱��ָ
Ԫ��
Ԫ��
���Ԫ�ء����ӡ�����������һ��ȱ�⣬���ܻ��еļ�����
��״���״�
��״���״�
�����״���״������ɡ�����
��4����������������ˮ����������
ʳ��
ʳ��
���ʳ�ס���ʳ��ˮ������
��5�������е�����������ˮ�����γ���Һ����
BD
BD
������ĸ��ţ���
A�������� B��ʳ�� C����� D������
��6�����õļҾӻ����������õ����
�ٷ���װ�������ڷ�һЩ����̿������װ�����ͷų��ļ�ȩ�������ж����壬�������û���̿��
����
����
�ԣ�
����ͼ1��ʾ�ġ�����Ϩ����һ�����͵ļ��������Ʒ��������Ϩ���Ӵ�������3-5���ը�����ͷŵķ�ĩ������ȼ�����ϣ�ͬʱ�ų�����ȼ���壬ʹ����Ϩ�𣮡�����Ϩ�������ԭ����
B
B
�����ţ���

A�������ȼ�� B��ʹȼ�������������� C������ȼ������Ż��
��7����ͼ2���������ִ������г�����һ�ֽ�ͨ���ߣ�
����������ӻ�����������ǵ���ʴ�������ԭ���Ǹ���
������ˮ
������ˮ
��
���ҹ����ƹ�ʹ�ó����Ҵ����ͣ������������м����������Ҵ�����Ϊ������ȼ�ϣ�
����������ȷ����
BC
BC
������ĸ����
A���Ҵ�������һ�����͵Ļ�����
B���Ҵ���ͨ����ʳ���͵ķ����Ƶ�
C��ʹ���Ҵ������ܼ����к�������ŷ�
D���Ҵ������Ͷ��ǿ�������Դ
������Ȼ����Ϊ��������ȼ����Ŀǰ���Ҵ����ᳫ�ģ���Ȼ������Ҫ�ɷ��Ǽ��飬����ȼ�յĻ�ѧ����ʽΪ
��
������������������Ҫ���ֽ�����������������������������ҵ�������ļ�Ҫ�������£�
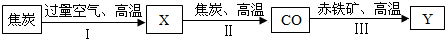
��д�������Ļ�ѧ��Ӧ����ʽ
��
��
��


 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�