
����С���ͬѧΪ��̽��ʵ���ҵ�һ�ֺ�ɫ��ĩ��һƿ��ǩ�������ɫ��Һ����ͼ��������ʲô���ʣ����벢���ʵ�������֤������������ǵ�̽����
���������⡿���Ѻ�ɫ��ĩ����ɫ��Һ���ʱ�������������ݡ�
���������ϡ����л�ѧʵ���ҳ����ĺ�ɫ��ĩ��CuO��MnO2��Fe3O4�����ۡ�̿�۵ȡ�
��������衿��ɫ��ĩ�� ����ɫ��Һ�� ����ֻдһ�ּ��裩

�����ʵ�顿����ʢ��������ɫ��ĩ���Թ��м��������Լ�ƿ�е���ɫ��Һ�������Թ��ռ����壻�ۼ������壨д������������
[ʵ������] --------------------------------
��ʵ����ۡ���1����ɫ��ĩ����ɫ��Һ���ʱ������Ӧ�Ļ�ѧ����ʽ�� ��
��2���������� ��ԭ���� ��
 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
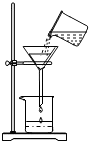 ��2012?������һģ��ij����С���ͬѧΪ�ⶨһƿBaCl2��Һ���ʵ�������������������ʵ�飬��ش��й����⣺
��2012?������һģ��ij����С���ͬѧΪ�ⶨһƿBaCl2��Һ���ʵ�������������������ʵ�飬��ش��й����⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com