�⣺��1������ͭ�е�����������������ӣ���ʾΪ��SO
42-���������е��������������ӣ���ʾΪ��Al
3+��θҺ�к��е�һ���������ᣬ�仯ѧʽΪHCl�������ڲ�������ֽ����֯��ϴ�Ӽ���һ������̼���ƣ��仯ѧʽΪNa
2CO
3��
��2��һ�ְ���ɫ�Ĺ���ȼ�ղ��������İ����Ǻ��ף��仯ѧʽΪP��
��3���н���ͭ�μӵ��û���Ӧ��������ԭ����ͭ����ѧ����ʽΪ��H
2+CuO

Cu+H
2O��
��4���г������ɵ��кͷ�Ӧ���������������ᷴӦ�������ᱵ������ˮ����ѧ����ʽΪ��Ba��OH��
2+H
2SO
4=BaSO
4��+2H
2O��
��5��ʵ������һ�ֹ����һ��Һ������������˫��ˮ�Ͷ������̣���ѧ����ʽΪ2H
2O
2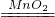
2H
2O+O
2����
�ʴ�Ϊ����1��SO
42-��Al
3+��HCl����2��P����3��H
2+CuO

Cu+H
2O����4��Ba��OH��
2+H
2SO
4=BaSO
4��+2H
2O����5��2H
2O
2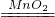
2H
2O+O
2����
��������1������ͭ�е�����������������ӣ��������е��������������ӣ�θҺ�к��е�һ��������������ڲ�������ֽ����֯��ϴ�Ӽ���һ������̼���ƣ�
��2��һ�ְ���ɫ�Ĺ���ȼ�ղ��������İ����Ǻ��ף�
��3���н���ͭ�μӵ��û���Ӧ��������ԭ����ͭ��
��4���г������ɵ��кͷ�Ӧ���������������ᷴӦ�������ᱵ������ˮ��
��5��ʵ������һ�ֹ����һ��Һ������������˫��ˮ�Ͷ������̣�
������������Ҫ�����������ض���������д��ѧʽ����д��ѧ����ʽ����д��ѧ����ʱһ��Ҫ�淶��ȷ��

 Cu+H2O��
Cu+H2O��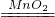 2H2O+O2����
2H2O+O2���� Cu+H2O����4��Ba��OH��2+H2SO4=BaSO4��+2H2O����5��2H2O2
Cu+H2O����4��Ba��OH��2+H2SO4=BaSO4��+2H2O����5��2H2O2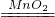 2H2O+O2����
2H2O+O2����

 ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�