��2013?��������ģ���ᡢ��κ��������֪ʶ�dz��л�ѧ��Ҫ�Ļ���֪ʶ��������ͬѧ�Ƕ����֪ʶ����̽���Ĺ��̣�
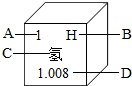
��1����ͼ������ж�������Ԫ�أ���ͼ������Ԫ�����ڱ��е�һЩ��Ϣ�����ж�ͼ����Ϣ�������Ӧ�ô������
��
��
��
��A��ʾ��ԭ�Ӻ��к���һ������ ��B��Ԫ�صķ��� ��C��Ԫ�ص����� ��D��ԭ�ӵ�����
��2�����ᣬ������������ᶼ�����ƵĻ�ѧ���ʣ��磺���Ƕ���ʹ���ָʾ����ɫ�����Ƕ�
��Ӧ�����κ�ˮ
��Ӧ�����κ�ˮ
��Ũ������
��ˮ��
��ˮ��
���ʿ��������������Ũ����ܸ��ﰱ������Ϊ�����������ᷴӦ����������泥��÷�Ӧ�Ļ�ѧ����ʽΪ
NH3+H2SO4�TNH4HSO4
NH3+H2SO4�TNH4HSO4
��3���������ơ��Ȼ��ơ�����ͭ�����ʵ�ˮ��Һ�ܵ��磬�������ǵĹ���ȱ���ܵ��磬����Ϊʲô��
��4��ˮ����Ҫ����������þ��̼�����ɵĻ���ͬѧ�Ƿֱ���ϡ�����ϡ��������ȥˮ����������ϡ����ܿ���ܽ�ˮ����ȥ��������ϡ�����ˮ��ʱ����Ӧ����ֹͣ������Ϊϡ����ܽ�ˮ����ȫ������ԭ����
̼��������ᷴӦ���ɵ����������ˮ���谭��Ӧ�Ľ���
̼��������ᷴӦ���ɵ����������ˮ���谭��Ӧ�Ľ���
��5��Ϊ�˲ⶨˮ���е�̼��Ƶĺ�����ͬѧ�dz�ȡ10.0gˮ�������ձ��У���4�ν�ϡ�������ձ��м��룬����������±���
|
δ��ϡ����ʱ |
��һ�μ���10.0gϡ���� |
�ڶ��μ���10.0gϡ���� |
�����μ���10.0gϡ���� |
���Ĵμ���10.0gϡ���� |
| �ձ������������� |
10.0g |
18.9g |
27.8g |
36.7g |
46.7g |
����㣺�ٷ�Ӧ���ɶ�����̼����������ˮ���к���̼��Ƶ�����������




 ��2013?��������ģ���ᡢ��κ��������֪ʶ�dz��л�ѧ��Ҫ�Ļ���֪ʶ��������ͬѧ�Ƕ����֪ʶ����̽���Ĺ��̣�
��2013?��������ģ���ᡢ��κ��������֪ʶ�dz��л�ѧ��Ҫ�Ļ���֪ʶ��������ͬѧ�Ƕ����֪ʶ����̽���Ĺ��̣� ��2013?��ɽ����ͼ��A��B��C��D��E�dz��л�ѧ�г��������ʣ�ת����ϵ�����в���������ͷ�Ӧ�����Ѻ��ԣ�����֪B�Ǻ�ɫ���塢��Է�������Ϊ80��E����Ȼ������Ҫ�ɷݣ���ش��������⣺��1��B��
��2013?��ɽ����ͼ��A��B��C��D��E�dz��л�ѧ�г��������ʣ�ת����ϵ�����в���������ͷ�Ӧ�����Ѻ��ԣ�����֪B�Ǻ�ɫ���塢��Է�������Ϊ80��E����Ȼ������Ҫ�ɷݣ���ش��������⣺��1��B��