
 ����H2SO4��CuSO4�Ļ����Һ��Ϊ�˷��������Һ��H2SO4��CuSO4�������������������ʵ�鷽����ȡ�ķݻ����Һ��100g���ֱ������뵽50g��100g��150g��200gijNaOH��Һ�У��������ʵ�����������
����H2SO4��CuSO4�Ļ����Һ��Ϊ�˷��������Һ��H2SO4��CuSO4�������������������ʵ�鷽����ȡ�ķݻ����Һ��100g���ֱ������뵽50g��100g��150g��200gijNaOH��Һ�У��������ʵ�����������| �ڢ��� | �ڢ��� | �ڢ��� | �ڢ��� | |
| NaOH��Һ������/g | 50 | 100 | 150 | 200 |
| ���ɳ���������/g | 0 | 2.45 | 7.35 | 9.8 |
| 160 |
| x |
| 98 |
| 9.8g |
| 80 |
| y |
| 98 |
| 9.8g |
| 16g |
| 100g |
| 8g |
| 100g |
| 98 |
| z |
| 80 |
| 75g��8% |
| 7.35g |
| 100g |



 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
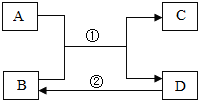 A��B��C��D�dz��л�ѧ�������ʣ�����֮�������·�Ӧ��ת����ϵ����Ӧ����δ�������
A��B��C��D�dz��л�ѧ�������ʣ�����֮�������·�Ӧ��ת����ϵ����Ӧ����δ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
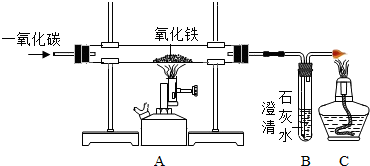
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com