���㣺ˮ�ľ���,�����ijɷּ����ɷֵ��������,���ˮʵ��,Ӳˮ����ˮ,�Ͻ���Ͻ������,������ʴ�������������,̼���ʵ��������ʼ���;,��ѧʽ����д������,����ȼ�ϵ�ʹ������Ի�����Ӱ��,����ԭ���ͷ���
ר�⣺��ѧ����������غ㶨��,������ˮ,�������������,��ѧ������
��������1�����ݻ���̿���������ԣ���������ζ��ɫ�أ�������н���ˮ��Ӳ�ȣ�
��2������̿���������ԣ���п��Խ���ˮ��Ӳ�ȣ�
��3�����ݻ�ѧʽ����д������գ�����Ԫ������ߣ�����Ԫ�����ұߣ��������ϼ۴�����Ϊ�㣻
��4����������ʴ�������ش�
��7�����ݴ�����ͻ����Ķ��������
��8�����ݺϽ�Ķ��������
��9������ȼ����Ҫ���������н��
��10������ȼ�ղ����˶�����̼��ˮ�����Ծݴ˽��
��11�����ݻ�������Ԫ�صĻ��ϼ�ԭ�������Ȼ���Ļ�ѧʽ�жϳ���Ԫ�صĻ��ϼۣ���д��������Ļ�ѧʽ���ɣ�
��12���ٸ��� �����еijɷ�������������㣬��Լռ21%����Լռ78%�����н��
�ڿ������˹�������������ʸɱ�����������̼����
��13����ˮ�м�������������������Ƶ�Ŀ������ǿˮ�ĵ����ԣ�
��14������ɭ�ֻ���ʱ���Բ�ȡ�ķ�����ԭ���ǣ���ͨ����������Դ������������ϻ�������Ļ���ʱӦ���жϵ�Դ���ٽ������
����⣺
��1������̿���������ԣ���������ζ��ɫ�أ�������ˮ�ھ��������г��û���̿����ɫ�غ���ζ��������н���ˮ��Ӳ�ȣ�
��2����ˮ���о���ʹ�û���̿����Ҫ�����û���̿�������ԣ������м��ܽ���ˮ��Ӳ�ȣ�����ɱ���������Ƿ�������У�
��3������Ҫ�����������ʽ���ڣ�������Ԫ�صĻ��ϼ���+4�ۣ���������Ļ�ѧʽΪ TiO
2��
��4������ʴ�������ǣ�����������ˮͬʱ�Ӵ���
��7��������ͻ������б��Ǹ��ݴ�����ͻ����ĸ�����ص㣬����ij���ʽ��б������ƶϣ����������Ǵ����ﻹ�ǻ����Ӻ�۽Ƕ���˵����Ҫ�������м������ʣ�����һ�ֵ��Ǵ�������ж��ֵ��ǻ��������к��ж������壬���Կ����жϿ���Ϊ������ֲ��Ĺ�������ܹ��ṩ������
��8���Ͻ�����ij�ֽ����м����ۺ�ijЩ������������ǽ����������γɵľ��н������Ե����ʣ�����DZȽϾ��ȵģ�������ʯ��һ�ֺ�����Ԫ�صĿ����ʣ��������н������ԣ��ʲ����ںϽ𣬹�ѡc��
��9������ȼ����Ҫ��������֪�����������һ���Ż𣬿��ù��Ǹ����������˸���������ԭ����
��10������ȼ�ղ����˶�����̼��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��CH
4+2O
22H
2O+CO
2��
��11���ڻ��������������ϼ۵Ĵ�����Ϊ�㣬���Ȼ�������Ԫ�صĻ��ϼ�Ϊ-1��
����Ԫ�صĻ��ϼ�Ϊx����
x+��-1����3=0��
��ã�x=+3��
����Ԫ�صĻ��ϼ�Ϊ+3����Ԫ�صĻ��ϼ�Ϊ-2�����ݻ��ϼ�ԭ�����д��������Ļ�ѧʽΪ��Eu
2O
3��
��12���ٸ��� �����еijɷ�������������㣬��Լռ21%����Լռ78%�����Կ����к�������������N
2��
�ڿ������˹�������������ʸɱ�����������̼������ѧʽΪ��CO
2��
��13����ˮ�м�������������������Ƶ�Ŀ������ǿˮ�ĵ����ԣ�
��14������ɭ�ֻ���ʱ���Բ�ȡ�ķ�����ԭ���ǣ���ͨ����������Դ������������ϻ�������Ļ���ʱӦ���жϵ�Դ���ٽ�����𣻹ʴ�Ϊ��1������̿��������У�
��2���������������
��3��TiO
2��
��4������������ˮͬʱ�Ӵ���
��7������������
��8��c��
��9������������
��10��CH
4+2O
22H
2O+CO
2��
��11��Eu
2O
3��
��12����N
2����CO
2 ��
��13����ǿˮ�ĵ����ԣ�
��14����ͨ����������Դ���жϵ�Դ��
���������⿼��֪ʶ��϶࣬���ʵ����ʾ������ʵ���;�����ճ�����ѧ���ʵ����ʺ���;����ȷ��������Ĺؼ���Ҫ�����������Ŀ�����ȣ�Ҫ�������ջ�������ͻ���ԭ����Ȼ��ϸ�µط������⣨��ͼ����Ϣ���ȸ�����Ϣ��Դ����ϸ�ĵ�̽��������������ĿҪ����������ѡ����ɣ�




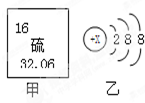 ͼ����ijԪ����Ԫ�����ڱ��еIJ�����Ϣ��ͼ���Ǹ�Ԫ�ص�һ�����ӽṹʾ��ͼ��
ͼ����ijԪ����Ԫ�����ڱ��еIJ�����Ϣ��ͼ���Ǹ�Ԫ�ص�һ�����ӽṹʾ��ͼ��