
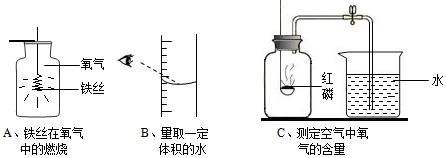
·ÖĪö £Ø1£©øł¾Ż“Ł½ųæÉČ¼ĪļČ¼Éյķ½·ØÓŠ£ŗŌö“óæÉČ¼ĪļÓėŃõĘųµÄ½Ó“„Ć껿»ņŌö“óŃõĘųµÄÅØ¶Č£¬½ųŠŠ·ÖĪö½ā“š£®
£Ø2£©ĢģČ»ĘųµÄÖ÷ŅŖ³É·ÖŹĒ¼×Ķé£¬Č¼ÉÕÉś³É¶žŃõ»ÆĢ¼ŗĶĖ®£¬Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½¼“æÉ£®
£Ø3£©Õż³£ÓźĖ®µÄpHŌ¼ĪŖ5.6£¬ĖįÓźŹĒÖøČÜŅŗpHŠ”ÓŚ5.6µÄÓźĖ®£»ĖįÓźÖ÷ŅŖÓÉ»ÆŹÆČ¼ĮĻČ¼ÉÕ²śÉśµÄ¶žŃõ»ÆĮņ”¢µŖŃõ»ÆĪļµČĖįŠŌĘųĢ壬¾¹żø“ŌӵēóĘų»Æѧ·“Ó¦£¬±»ÓźĖ®ĪüŹÕČܽā¶ų³É£¬¾Ż“Ė½ųŠŠ·ÖĪö½ā“š£®
½ā“š ½ā£ŗ£Ø1£©Éś»īÖŠ£¬ĪŅĆĒ³£³£½«Ćŗ¼Ó¹¤³É·äĪŃĆŗד£¬Ōö“óĮĖĆŗÓėŃõĘųµÄ½Ó“„Ć껿£¬ÄÜŹ¹Ćŗ³ä·ÖČ¼ÉÕ£®
£Ø2£©ĢģČ»ĘųµÄÖ÷ŅŖ³É·ÖŹĒ¼×Ķ飬¼×ĶéŌŚµćČ¼Ģõ¼žĻĀČ¼ÉÕÉś³É¶žŃõ»ÆĢ¼ŗĶĖ®£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖCH4+2O2$\frac{\underline{\;µćČ¼\;}}{\;}$CO2+2H2O£®
£Ø3£©»ÆŹÆČ¼ĮĻŌŚČ¼ÉÕ¹ż³ĢÖŠ»į²śÉśø÷ÖÖ·ĻĘų£¬ÄÜŠĪ³ÉĖįÓźµÄĘųĢåŹĒ¶žŃõ»ÆĮņŗĶ¶žŃõ»ÆµŖ£®
¹Ź“š°øĪŖ£ŗ£Ø1£©Ōö“óĮĖĆŗÓėŃõĘųµÄ½Ó“„Ć껿£¬ĄūÓŚĆŗ³ä·ÖČ¼ÉÕ£»£Ø2£©CH4+2O2$\frac{\underline{\;µćČ¼\;}}{\;}$CO2+2H2O£»£Ø3£©¶žŃõ»ÆĮņ£®
µćĘĄ ±¾ĢāÄŃ¶Č²»“ó£¬ÕĘĪÕ“Ł½ųæÉČ¼ĪļČ¼Éյķ½·Ø”¢»Æѧ·½³ĢŹ½µÄŹéŠ“·½·Ø”¢ĮĖ½āĖįÓźµÄŠĪ³ÉŌŅņŹĒÕżČ·½ā“š±¾ĢāµÄ¹Ų¼ü£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ö»ÓŠ¢Ł | B£® | Ö»ÓŠ¢Ś¢Ü | C£® | Ö»ÓŠ¢Ś¢Ū¢Ü | D£® | Č«²æ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
| ¼ÓČėµÄŹŌ¼Į | ²āµĆŹż¾Ż | |
| ·½·Ø1 | ×ćĮæBaCl2ČÜŅŗ | BaCO3³Įµķ1.97g |
| ·½·Ø2 | ×ćĮæĻ”ĮņĖį | CO2ĘųĢå0.44g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ½«ĘųĢå·Ö±šĶØČė³ĪĒåŹÆ»ŅĖ® | B£® | ½«ĘųĢå·Ö±šĶØČėŹÆČļČÜŅŗ | ||
| C£® | ŹŌįżČżÖÖĘųĢåµÄČܽāŠŌ | D£® | ÓĆČ¼×ŵÄľĢõ·Öe½Ó“„µ¼³öµÄĘųĢå |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | æŖ·¢ŠĀÄÜŌ“£ØĢ«ŃōÄÜ”¢³±Ļ«ÄÜµČ£©£¬¼õÉŁ»ÆŹÆČ¼ĮĻµÄŹ¹ÓĆ | |
| B£® | ĻŽÖĘ»Æ¹¤·¢Õ¹£¬¹ŲĶ£ĖłÓŠ»Æ¹¤ĘóŅµ£¬Ļū³żĪŪČ¾Ō“Ķ· | |
| C£® | ÓƵŚĖÄ“śLEDĀĢÉ«¹āŌ““śĢę°×³ćµĘ | |
| D£® | Ģį³«³Ė×ų¹«¹²½»Ķع¤¾ß£¬Ęļ×ŌŠŠ³µ»ņ²½ŠŠµČ³öĻÖ·½Ź½ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µ„ÖŹŹĒÓÉŅ»ÖÖŌŖĖŲ×é³ÉµÄ£¬ĖłŅŌŅ»ÖÖŌŖĖŲÖ»ÄÜ×é³ÉŅ»ÖÖµ„ÖŹ | |
| B£® | Ńõ»ÆĪļÖŠŅ»¶Øŗ¬ÓŠŃõŌŖĖŲ£¬ĖłŅŌŗ¬ÓŠŃõŌŖĖŲµÄ»ÆŗĻĪļŅ»¶ØŹĒŃõ»ÆĪļ | |
| C£® | ĀĮ±ķĆęµÄŃõ»ÆĀĮ±”ĤÄÜĘš±£»¤×÷ÓĆ£¬ŌņĢś±ķĆęµÄŃõ»ÆĢśŅ²Ęš±£»¤×÷ÓĆ | |
| D£® | »ÆŗĻĪļŹĒÓɲ»Ķ¬ÖÖŌŖĖŲ×é³ÉµÄ“æ¾»Īļ£¬ŌņÖ»ŗ¬Ņ»ÖÖŌŖĖŲµÄĪļÖŹŅ»¶Ø²»ŹĒ»ÆŗĻĪļ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com