
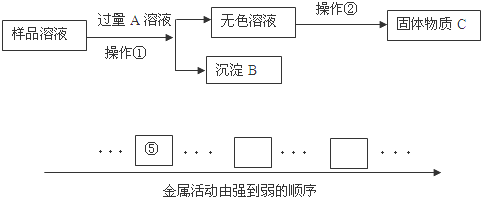
(6��)�ݱ�����һЩ������ũ������ֲˮ����ͬʱ���㣬 �ﵽˮ����������յ�Ŀ�ġ��밴Ҫ��ش��������⣺����������Ӫ���ض�������������ǣ�1��__________________________ ������ʱ�õ�������������˿�Ƴɣ��������ںϳɲ����еģ�2) ���������ˮʱ���õ�ˮ�ã�ˮ����ͨ�������������ù���ʵ�ֵ�����ת���ǣ�3��______����ˮ�����ֵ�������Ӧʩ�ӵĻ��������ǣ�4��___________________________��ˮ������������Ҳ��Ҫʩ�ӵ��ʣ����������������ײ����ֽ��������ֿ����н϶�������һ�ֳ���Һ�壬�䷴Ӧ�Ļ�ѧ����ʽΪ��5)___________________________����Ӧǰ��Ԫ�ػ��ϼ۵ı仯Ϊ��6��_______��
��1����1���ǹ���ϸ���Ļ������ʣ��ǻ�����������������֯����Ҫԭ�ϣ����ṩ������
��2���ϳ���ά ��3������ת��Ϊ��е�� ��4���ط�
��5��2NH4NO3ײ�� 2N2 �� +O2 ��+ 4H2O ��6��-3�� +5�۱�Ϊ0��
���������������1�� ����������Ӫ�����ǵ����ʣ���������������ǣ��ǹ���ϸ���Ļ������ʣ��ǻ�����������������֯����Ҫԭ�ϣ����ṩ������
��2�� �������ںϳɲ����еĺϳ���ά
��3�� ˮ����ͨ�������¹������ù���ʵ�ֵ�����ת���ǣ�����ת��Ϊ��е��
��4���طʵ����ã���ʹ����������׳�����˴�Ӳ����������׳�ѣ�����ˮ�����ֵ�������Ӧʩ�ӵĻ����Ǽط�
��5�����������������ײ����ֽ��������ֿ����н϶�����壨����������������һ�ֳ���Һ�壨��ˮ�����䷴Ӧ�Ļ�ѧ����ʽΪ��2NH4NO3ײ�� 2N2 �� +O2 ��+ 4H2O
��6������Ԫ�ػ��ϼ۵�һ����ɣ��ڻ������У��������ϼ۵Ĵ�����Ϊ0��������NH4NO3�е�2��ԭ����NH4�Ļ��ϼ�Ϊ+1��H�Ļ��ϼ�Ϊ+1����N�Ļ��ϼ�Ϊ-3��NO3�Ļ��ϼ�Ϊ-1��O�Ļ��ϼ�Ϊ-2����N�Ļ��ϼ�Ϊ+5�����ڵ�����Ԫ�صĻ��ϼ�Ϊ0��
���㣺������Ҫ��Ӫ�����ʣ��ϳɲ��ϣ���ѧ���ϣ���ѧ����ʽ����д�����ϼ۵�һ�����


 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
��ȤС���ͬѧѧϰ�˽��������ʺ���ʵ�����ҵ���һ������ɫ����R������̽����
��1��������RͶ��ϡ�����У���������������ð������ý���R�ڽ������˳������������ ��ѡ�ǰ�桱���桱����
��2��Ϊ�˱ȽϽ���R�����Ļ��ǿ������ȤС��������з�������ʵ�飬������±���
| ���һ�ֲ��� | ��֤���� | ���� | ���� |
| ����R�Ļ�Ա��� | ������RͶ��������Һ�� | ����R�����к�ɫ���������� | ����������ѡ���ȷ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(6�֣��������������ѡ���ʵ���������գ�����ĸ��ţ���
| A���ɱ� | B���ƾ� | C������ | D������̿ E��ʯ��ʯ F��ά����C |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ������ϢϢ��أ���������ѧ��ѧ֪ʶ�ش����������е����⡣
��1��С����ѧһ�����ž��ŵ����㣬��֤���������� ���ʡ�
��2��С������������ϴ�·���ˮ�м������ˮ�����������״���������Ϊ��ʱ��ˮΪ ���Ӳˮ������ˮ������
��3������ˮ����ˮ�����г��û���̿������̿����Ҫ������ ��
��4��ϴ�Ӽ�����ϴ�;��ϵ����ۣ�������Ϊϴ�Ӽ����� ���ܡ�
��5����ʯ�ҳ�����ʳƷ���������ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������Ҫ��ԭ���ᴿ����������߲�Ʒ���������ⶨij�Ȼ�����Ʒ�л��������ƣ�Ϊ�˳�ȥ���ʲ��Ƶô������Ȼ��ƹ��壬��ʵ�����о���ijѧ����Ʒ������£�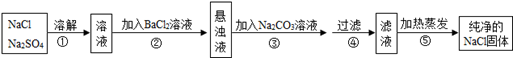
�ش���������
��1���������ܷ������ᱵ��Һ��˵������ ��
��2�����в����ں�����ж��������ѳ����������� ��
��3�������۵�Ŀ���� ���ۡ��ܲ���˳���ܷ����������� ��
��4������Ʒ����Ƿ����ܣ�˵������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾװ�õ�U�ι������ڹ̶�һС�Թܣ�
��1�����Ҳ���ڹҵ��ǵ��з�̪��Һ����ֽ������С�Թ��еμ�Ũ��ˮ���ɹ۲쵽��ֽ������ɫ�仯���� ����������С�Թ��м��������ƹ��壬�ٽ�������ʵ�����������ڸ��̵�ʱ���ڹ۲쵽��ֽ������ͬ�ı仯�����ܵ�ԭ������ ����
��2�����Ҳ���ڹҵ���ʯ����Һ���ݹ�����ֽ��������һ����ʪ��ģ�һ�������ɵģ���װ��̼���Ʒ�ĩ��С�Թ��еμ�ϡ���ᣬд��С�Թ��з�����Ӧ�Ļ�ѧ����ʽ��������֤��������̼����ˮ��Ӧ������������Ҫ���������Ա������Ͳ��U�ι���ע��������������Һ��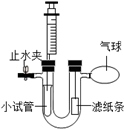
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ȥNaCl�л��е�ϸɰ�������õõ��IJ���NaCl������Һ��ʵ�������ͼ��ʾ��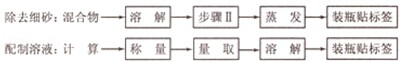
��1�������IJ����������� ����
��2������ʱ�����õ�����������̨������Ȧ���� ��������Ͳ�������
��3������50g��������Ϊ6%��NaCl��Һ����NaCl�� ��g��
��4�������������������Һ����������Ӱ������� ��
A���õ���NaClδ��ȫ����
B������Ͳ��ȡˮʱ�����Ӷ���
C����õ���Һװƿ���ձ�������Һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��4�֣�ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã���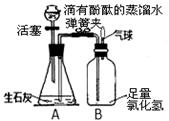
��1���ӷ�Һ©������һ����Һ�壬�����رջ��������ɼУ�A���ܹ۲쵽�������� ��
��2��δ�ָ������£����رյ��ɼУ�Bƿ�в��������ԭ����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��4�֣��±��Ǽ��ּ����������й���Ϣ��
| �������� | ����� | ������Ư | Ư�� |
| ��Ч�ɷ� | HCl | H2O2 | NaClO |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com