
| µŚ1“Ī | µŚ2“Ī | µŚ3“Ī | µŚ4“Ī | µŚ5“Ī | |
| ¼ÓČėĻ”ŃĪĖįµÄÖŹĮæ/g | 20 | 20 | 20 | 20 | 20 |
| Éś³ÉĘųĢåµÄ×ÜÖŹĮæ/g | 1.1 | 2.2 | m | 4.4 | 4.4 |
·ÖĪö Ģ¼ĖįøĘŗĶĻ”ŃĪĖį·“Ӧɜ³ÉĀČ»ÆøĘ”¢Ė®ŗĶ¶žŃõ»ÆĢ¼£¬øł¾Ż±ķÖŠĢį¹©µÄŹż¾ŻŗĶ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĄūÓĆ½ųŠŠĻą¹Ų·½ĆęµÄ¼ĘĖćŗĶÅŠ¶Ļ£®
½ā“š ½ā£ŗ£Ø1£©·ÖĪå“Ī¼ÓČėŃĪĖįŗó£¬Ē°Į½“Ī£¬Ćæ“Ī¶¼Ōö¼Ó1.1g¶žŃõ»ÆĢ¼£¬µŚĖÄ”¢Īå¶¼Éś³É4.4g¶žŃõ»ÆĢ¼£¬ĖµĆ÷µŚČż“ĪƻӊĶźČ«·“Ó¦£¬Ņ²Ōö¼Ó1.1g£¬ĖłŅŌmÖµŹĒ3.3g£»
£Ø2£©Éčѳʷ֊Ģ¼ĖįøʵÄÖŹĮæĪŖx£¬Ļ”ŃĪĖįČÜŅŗµÄČÜÖŹµÄÖŹĮæĪŖy£¬øł¾ŻĢāÄæÖŠĢį¹©µÄŠÅĻ¢æÉÖŖ£¬Ģ¼ĖįøĘÓėŃĪĖį·“Ó¦ĶźČ«Ź±ĖłÉś³ÉµÄ¶žŃõ»ÆĢ¼µÄÖŹĮæĪŖ4.4g
CaCO3+2HCl=CaCl2+CO2ӟ+H2O
100 73 44
x y 4.4g
$\frac{100}{x}$=$\frac{73}{y}$=$\frac{44}{4.4g}$
x=10g
y=7.3g
£Ø3£©Ļ”ŃĪĖįČÜŅŗµÄČÜÖŹµÄÖŹĮæĪŖ7.3g£®
¹Ź“š°øĪŖ£ŗ£Ø1£©3.3£»
£Ø2£©10£»
£Ø3£©7.3£®
µćĘĄ ±¾ĢāÖ÷ŅŖæ¼²éѧɜŌĖÓĆ¼ŁÉč·ØŗĶ»Æѧ·½³ĢŹ½½ųŠŠ¼ĘĖćŗĶĶʶĻµÄÄÜĮ¦£¬Ķ¬Ź±æ¼²éĮĖ·ÖĪöŹż¾ŻµÄÄÜĮ¦£¬¼ĘĖ揱ŅŖ×¢Ņā¹ę·¶ŠŌŗĶ×¼Č·ŠŌ£®


 ŗ£µķæĪŹ±ŠĀ×÷Ņµ½š°ń¾ķĻµĮŠ“š°ø
ŗ£µķæĪŹ±ŠĀ×÷Ņµ½š°ń¾ķĻµĮŠ“š°ø ĘŚÄ©½šÅĘ¾ķĻµĮŠ“š°ø
ĘŚÄ©½šÅĘ¾ķĻµĮŠ“š°ø ĒįĖÉæĪĢƱź×¼Į·ĻµĮŠ“š°ø
ĒįĖÉæĪĢƱź×¼Į·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | Ė®ĆŗĘųµÄ³É·ÖŹĒŅ»Ńõ»ÆĢ¼ŗĶŃõĘų | |
| B£® | ·“Ó¦Ē°ŗóø÷ŌŖĖŲµÄ»ÆŗĻ¼Ū¾ł²»±ä | |
| C£® | øĆ·“Ó¦ÖŠŗ¬ĒāŌŖĖŲµÄ»ÆŗĻĪļÓŠ3ÖÖ | |
| D£® | øĆ·“Ó¦»Æѧ·½³ĢŹ½ÖŠ¼×ĶéŗĶĖ®µÄ¼ĘĮæŹżÖ®±ČĪŖ1£ŗ1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĢśĖæŌŚæÕĘųÖŠ¾ēĮŅČ¼ÉÕ£¬»šŠĒĖÄÉä | B£® | NaOHČÜÓŚĖ®£¬ĪĀ¶ČĻŌÖųÉĻÉż | ||
| C£® | ·ÓĢŖÖŠµĪČė°±Ė®£¬ČÜŅŗ±äĪŖĄ¶É« | D£® | ŌŚČ¼ÉÕ³×ÄŚ×ĘÉÕĆę·Ū£¬·¢Éś±¬ÕØ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | Šč¼ų±šµÄĪļÖŹ | ·½·Ø”¢ĻÖĻó¼°½įĀŪ |
| A | ±£ĻÕĖæÓėĪŁĖæ | ¼ÓČČ£¬ĻČČŪ»ÆµÄŹĒ±£ĻÕĖæ |
| B | Ó²Ė®ŗĶČķĖ® | ¼ÓČė·ŹŌķĖ®£¬Õńµ“£¬ÅŻÄ¶ąµÄŹĒÓ²Ė® |
| C | Ģś·ŪÓėĢ¼·Ū | æ“ŃÕÉ«£¬Ņų°×É«µÄŹĒĢś·Ū |
| D | Ź³ŃĪĖ®ŗĶÕōĮóĖ® | ³¢Ī¶µĄ£¬ÓŠĻĢĪ¶µÄŹĒŹ³ŃĪĖ® |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ČÜŅŗ¶¼ŹĒĪŽÉ«ĶøĆ÷µÄŅŗĢ壬ČēĀČ»ÆÄĘČÜŅŗ | |
| B£® | ĪļÖŹµÄČܽā¹ż³ĢĶس£»į°éĖę×ÅÄÜĮæµÄ±ä»Æ£¬ČēĒāŃõ»ÆÄĘČÜÓŚĖ®ĪüČČ | |
| C£® | ÅäÖĆČÜŅŗŹ±£¬½Į°čæÉŅŌŌö“ó¹ĢĢåĪļÖŹµÄČܽā¶Č | |
| D£® | 60”ꏱĻõĖį¼ŲµÄČܽā¶ČĪŖ110g£¬ĘäČÜŅŗÖŠČÜÖŹÓėČÜŅŗµÄÖŹĮæ±ČĪŖ11£ŗ21 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | 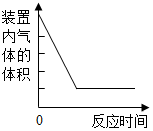 ²ā¶ØæÕĘųÖŠŃõĘųµÄŗ¬Įæ | |
| B£® | 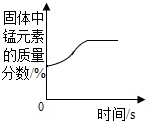 ¼ÓČČŅ»¶ØĮæµÄøßĆĢĖį¼Ų¹ĢĢå | |
| C£® | 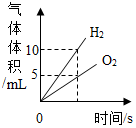 ½«Ė®ĶØÖ±Į÷µēŅ»¶ĪŹ±¼ä | |
| D£® | 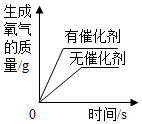 ÓƵČÖŹĮ攢µČÅØ¶ČµÄĖ«ŃõĖ®ÖĘČ”ŃõĘų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£®ÄÜŌ“ĪŹĢā | B£®ČĻŹ¶Į£×Ó |
| ¢ŁĢ«ŃōÄÜ”¢·ēÄÜŹĒĒå½ąÄÜŌ“ ¢Ś»ÆŹÆČ¼ĮĻŹĒ²»æÉŌŁÉśÄÜŌ“ ¢ŪĢģČ»ĘųĒå½ą»·±££¬Č”Ö®²»¾””¢ÓĆÖ®²»½ß | ¢ŁŌ×ӱȷÖ×ÓŠ” ¢Ś·Ö×ÓŌĖ¶ÆŌ×Ó²»ŌĖ¶Æ ¢Ū·Ö×ÓÓÉŌ×Ó¹¹³É |
| C£®»·¾³ĪŹĢā | D£®ŠŌÖŹÓėÓĆĶ¾ |
| ¢ŁæÕĘųÖŹĮæČÕ±ØÖŠĪŪČ¾ÖøŹżÖ÷ŅŖŹĒµŖ”¢ĮņŃõ»ÆĪļŗĶæÉĪüČėæÅĮ£Īļ ¢ŚĖ®ĪŪČ¾Ö÷ŅŖĄ“×Ō¹¤Ņµ”¢Å©ŅµŗĶÉś»īĪŪČ¾ ¢ŪŹ¹ÓĆ»ÆŹÆČ¼ĮĻ»į¶Ō»·¾³Ōģ³É²»Į¼Ó°Ļģ | ¢ŁCOÓŠ¶¾ŠŌ£¬æÉÓĆÓŚŅ±Į¶½šŹō ¢ŚŃõĘųÄÜÖśČ¼£¬æÉ×÷Č¼ĮĻ ¢ŪĢśÓŠµ¼ČČŠŌ£¬æÉ×÷³ÉĢś¹ų |
| A£® | A”¢ | B£® | B”¢ | C£® | C”¢ | D£® | D”¢ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com