С����ⶨCu-Zn�Ͻ��Cu-Ag�Ͻ���ͭ������������ʵ����ֻ�ṩ��һƿδ������������������ϡ����ͱ�Ҫ��������
��1������Ϊ���ܲ��ͭ�����������ĺϽ���______�Ͻ�
��2��С��ȡ�úϽ�ķ�ĩ32.5g���������������ַ�Ӧ���ⶨ������0.4g���壬���������������úϽ���ͭ������������
��3��С����������Ӧ���������������Ϊ150g������������������Ӧ����Һ��������Ϊ���ʵ����������Ƕ��٣�
���𰸡�
��������1����ΪCu��Ag�Ľ�����Զ��ڣ�H�������Զ�������ϡ���ᷢ���û���Ӧ����Zn�Ľ�������ڣ�H��ǰ������ϡ���ᷢ���û���Ӧ���������ͭ���������ݴ˴��⣮
��2������п��ϡ���ᷴӦ�Ļ�ѧ����ʽ�����ɵ������������г�����ʽ���Ϳɼ�������뷴Ӧ��Zn������������ZnCl
2��������Ȼ���������������ʽ�Ϳɼ�����úϽ���ͭ�����������������������-п������=�������ͭ��������
��3������������������=
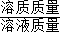
×100%���㼴�ɣ�����Һ����=�������������+�����ᷴӦ��Zn������-���������������
����⣺��1����ΪCu��Ag�Ľ�����Զ��ڣ�H�������Զ�������ϡ���ᷢ���û���Ӧ����Zn�Ľ�������ڣ�H��ǰ������ϡ���ᷢ���û���Ӧ���������ͭ����������ѡCu-Zn�Ͻ�
��2���������ᷴӦ��Zn������Ϊx������ZnCl
2������Ϊy��
Zn+2HCl�TZnCl
2+H
2��
65 136 2
x y 0.4g
��
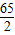
=

����֮�ã�x=13g��
��
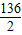
=
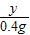
����֮�ã�y=27.2g��
�Ͻ���ͭ����������Ϊ��
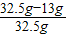
×100%=60%��
��3����Ӧ����Һ��������ΪZnCl
2��Ӧ����Һ��ZnCl
2������������
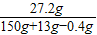
×100%=16.7%��
�𣺣�2���úϽ���ͭ����������Ϊ60%��
��3����Ӧ����Һ��ZnCl
2����������Ϊ16.7%��
������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ���м����������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������

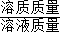 ×100%���㼴�ɣ�����Һ����=�������������+�����ᷴӦ��Zn������-���������������
×100%���㼴�ɣ�����Һ����=�������������+�����ᷴӦ��Zn������-���������������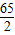 =
= ����֮�ã�x=13g��
����֮�ã�x=13g��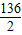 =
=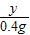 ����֮�ã�y=27.2g��
����֮�ã�y=27.2g��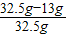 ×100%=60%��
×100%=60%��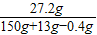 ×100%=16.7%��
×100%=16.7%��


