
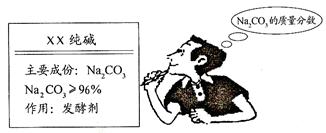
| | ʵ�鷽�� |
| Сǿ | ȷ��ȡ5.5 g������Ʒ�����ձ��У���ȡ��50�ˡ�14.6%��ϡ������μ��룬���պò��ٲ�������ֹͣ�μ�ϡ���ᣬ��ʣ��ϡ����25�ˡ� |
| С�� | ȷ��ȡ5.5 g������Ʒ������ƿ�У�����һ������������������ϡ���ᣬ������������������ͨ������������������Һ�У�����ͼ��ʾ�� ��������պ�������������Һ����������2.0�ˡ�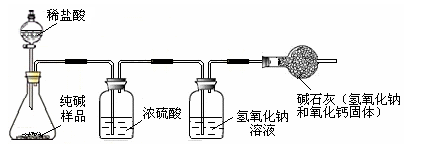 |
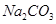 +2HCl="2NaCl+" H2O+ CO2��
+2HCl="2NaCl+" H2O+ CO2��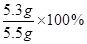 =96.4%��
=96.4%��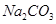 +2HCl="2NaCl+" H2O+ CO2��
+2HCl="2NaCl+" H2O+ CO2��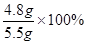 =87.6%��
=87.6%��

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
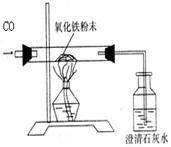
| | ��Ӧǰ | ��������ȫ��Ӧ�� |
| A�� | �����ܺ���Ʒ������43.7 g | �����ܺ������ʵ�����41.3 g |
| B�� | ���ƿ�ͳ���ʯ��ˮ������180 g | ���ƿ��ƿ�����ʵ�����186.2 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��14.9% | B��65% | C��75% | D��80% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
 Si+ 4HCl�������Ҫ���56g�裨Si����������Ҫ�������ٿˣ�
Si+ 4HCl�������Ҫ���56g�裨Si����������Ҫ�������ٿˣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| ʱ��/s | 0 | 30 | 50 | 90 | 120 | 150 | 180 |
| ��������/g | 0 | 30 | 50 | 60 | 80 | 66 | 66 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| ʵ����� | 1 | 2 | 3 |
| ϡ����������g�� | 50 | 50 | 100 |
| ��Ʒ������g�� | 20 | 30 | 20 |
| ���������������g�� | 6.6 | 6.6 | 6.6 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com