
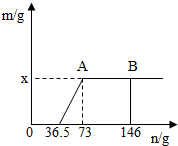
 һ�ձ���ʢ��Na2CO3��NaOH�Ļ��������м�����ˮ�ܽ��Ƴ���Һ�����μ�������������Ϊ10%��ϡ���ᣬ�ų������������m�������μ�ϡ�����������n���Ĺ�ϵ��ͼ��ʾ������������ͼʾ����������⣺
һ�ձ���ʢ��Na2CO3��NaOH�Ļ��������м�����ˮ�ܽ��Ƴ���Һ�����μ�������������Ϊ10%��ϡ���ᣬ�ų������������m�������μ�ϡ�����������n���Ĺ�ϵ��ͼ��ʾ������������ͼʾ����������⣺| 73 |
| 44 |
| (73-36.5)��10% |
| x |
| 36.5 |
| 58.5 |
| 73��10% |
| y |


 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
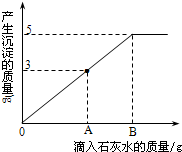 ��2011?���϶�ģ����һ�ձ���ʢ��Na2CO3��NaOH�Ļ����10g�������м���100gˮ������ȫ���ܽ⣮��������Һ�еμӳ���ʯ��ˮ���������������������ʯ��ˮ��������ϵ������ͼ��ʾ�����������ش��������⣺
��2011?���϶�ģ����һ�ձ���ʢ��Na2CO3��NaOH�Ļ����10g�������м���100gˮ������ȫ���ܽ⣮��������Һ�еμӳ���ʯ��ˮ���������������������ʯ��ˮ��������ϵ������ͼ��ʾ�����������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
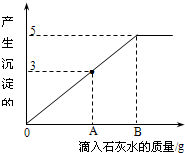 ��һ�ձ���ʢ��Na2CO3��NaOH�Ļ����10g�������м���100gˮ������ȫ���ܽ⣮��������Һ�еμӳ���ʯ��ˮ���������������������ʯ��ˮ��������ϵ������ͼ��ʾ�����������ش��������⣺
��һ�ձ���ʢ��Na2CO3��NaOH�Ļ����10g�������м���100gˮ������ȫ���ܽ⣮��������Һ�еμӳ���ʯ��ˮ���������������������ʯ��ˮ��������ϵ������ͼ��ʾ�����������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ����������Ƭ�����꼶ѧҵˮƽ���Ի�ѧ�Ծ����������� ���ͣ�������
��7�֣���һ�ձ���ʢ��Na2CO3��NaOH�Ļ����10g�������м���100gˮ������ȫ���ܽ⡣��������Һ�еμӳ���ʯ��ˮ���������������������ʯ��ˮ��������ϵ������ͼ��ʾ�����������ش��������⣺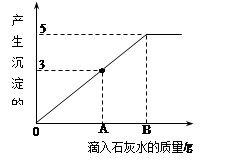
��1���ڵ���ʯ��ˮʱ��������������ɫ��______��
��2��������ʯ��ˮ��ͼ��A��ʱ���ձ�����Һ�ﺬ�е������ǣ�д��ѧʽ�� ��
��3�����㵱����ʯ��ˮ��ͼ��B��ʱ����Һ�����ʵ�����Ϊ���٣�����������ȷ��0.1g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�������������о��꼶Ƭ��ѧҵˮƽ���Ի�ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com