
 ��ͼ��ij��Ƭ��Ʒ�ı�ǩ��
��ͼ��ij��Ƭ��Ʒ�ı�ǩ������ ��1��������Է�������Ϊ���ɷ��ӵĸ�ԭ�ӵ����ԭ������֮�ͣ���������Ԫ�ص���������=$\frac{���ԭ��������ԭ�Ӹ���}{��Է�������}$��100%����������ijԪ�ص�����=�û��������������Ԫ�ص��������������з������
��2������̼��������ᷴӦ�Ļ�ѧ����ʽ���������غ㶨�ɣ����ٵ�������Ϊ���ɶ�����̼������������ж�����̼����������Ӧ�Ļ�ѧ����ʽ�����ÿƬ�к�̼��Ƶ��������벹�Ƽ���˵���Ƚ��ж��Ƿ���ʵ��
��� �⣺��1��̼��Ƶ���Է���������40+12+16��3+16=100��
̼����и�Ԫ�ص���������Ϊ$\frac{40}{100}��$100%=40%��
�������⣬ÿƬ��̼��ơ�1.96g��ÿƬ�����ٺ���Ԫ�ص�����Ϊ1.96g��40%=0.784g��
��2�����������غ㶨�ɣ����ɶ�����̼������Ϊ3g/Ƭ��5Ƭ+40.0g-50.6g=4.4g��
������ÿƬ��Ƭ��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x 4.4g
$\frac{100}{44}=\frac{x}{4.4g}$ x=10g
��ÿƬ�к�̼���10g��5=2g��2g��1.96g�����Ƭ��̼��Ƶĺ�����ע��ʵ��
�𣺣�1��100��40%��0.784g��
��2����4.4���ڸ�Ƭ��̼��Ƶĺ�����ע��ʵ��
���� �����ѶȲ������ո��ݻ�ѧ����ʽ�ļ����뻯ѧʽ�ļ��㼴����ȷ����⣬���������غ㶨�ɼ����������̼����������ȷ�����Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
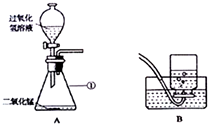 ��ͼ��ijͬѧ��Ƶ�ʵ������ȡ������ʵ��װ��ͼ����ش��������⣺
��ͼ��ijͬѧ��Ƶ�ʵ������ȡ������ʵ��װ��ͼ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ij���Ӻ�������������������������������������һ���������� | |
| B�� | �����������8�����ӵ����Ӷ���ϡ�������ԭ�� | |
| C�� | ��������ͬ������һ������ͬ��Ԫ�� | |
| D�� | ij���ʾ�������ֻ֪��һ��Ԫ�أ��������һ���ǵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������뵪���ķе㲻ͬ�����÷���Һ̬�������Ƶô������������˹����������仯 | |
| B�� | �����ڵ��¡���ѹ�������¿���ת��ΪҺ������ | |
| C�� | ������ֲ�������õ���Ҫ��Դ | |
| D�� | �����Ļ�ѧ���ʱȽϻ��ã����п�ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | O2 | B�� | H2 | C�� | H2O | D�� | O3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com