
| ��һ�� | �ڶ��� | ������ | |
| ��ȡ�������������/g | 10 | 10 | 15 |
| ����ϡ���������/g | 60 | 70 | 50 |
| ����CO2������/g | 3.3 | 3.3 | 3.3 |
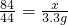 x=6.3g
x=6.3g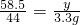 y=4.3875g
y=4.3875g =
= z=2.7375g
z=2.7375g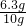 ��100%=63%
��100%=63%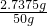 ��100%=5.48%
��100%=5.48%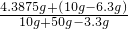 ��100%=14.25%
��100%=14.25%


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ѡ���װ�� | ʵ������ | ʵ����� |
AB����AC����ACB AB����AC����ACB |
��B������ǡ���C����������C��������B����롱 ��B������ǡ���C����������C��������B����롱 |
��Ʒ��������A |
| 106 |
| X |
| 197 |
| 19.7g |
| 10.6g |
| 12.0g |
| 106 |
| X |
| 197 |
| 19.7g |
| 10.6g |
| 12.0g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 NH3��+HCl����
NH3��+HCl����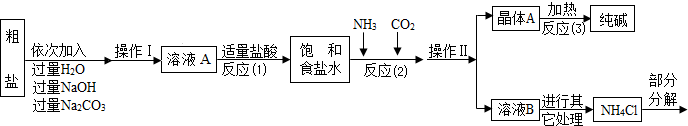
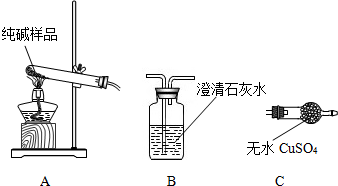
| ѡ���װ�� | ʵ������ | ʵ����� |
| ______ | ______ | ��Ʒ��������A |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
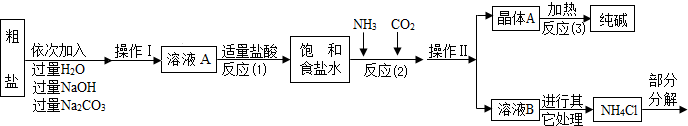
ijУ��ѧ��ȤС��ι��Ƽ���������Ϣ���������������о���
���������ϡ�
������ԭ�ϴ����к����������������ʣ�MgCl2��CaCl2�������������ʡ�
������ԭ����Ӧ�ƣ�NaCl+ NH3 + CO2 + H2O= NaHCO3��+ NH4Cl������þ���A����ʹ�������ȣ��ɷֽ��Ƶô�����ֳ����������
���Ȼ�立ֽ�Ļ�ѧ����ʽ��NH4Cl �� NH3��+HCl����
�ܲ���������������ͼ��ʾ��
 |
��1������ҺA�е�������NaCl�� �� ���ڲ����������Ϊ ��
��������NaOH��Һ�������dz�ȥ�����е� ��
��д������Na2CO3��Һ��������Ӧ�Ļ�ѧ����ʽ ��
��2���������������п�ѭ��ʹ�õ��� ������ţ���
A��CO2 B��NH3 C��HCl D��NaOH
�����̽��һ����3���پ���A���ȷֽ�Ļ�ѧ����ʽΪ ��
�����ʵ����鴿����Ʒ���Ƿ���о���A��������±���
�����̽��������4��ȡ������Ʒ��ˮ�ܽ⣬�����Һ�м������ϡHNO3���ٵμ�AgNO3��Һ���а�ɫ���������������ķ���ʽΪ ���ɴ�ȷ��������Ʒ��������NaCl��

| ѡ���װ�� | ʵ������ | ʵ����� |
| ��Ʒ��������A |
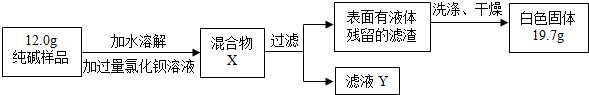
�����̽��������5��ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ�飺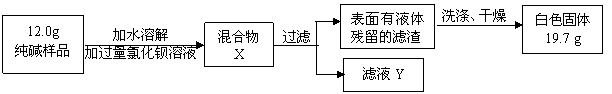
���жϼ����Ȼ�����Һ�Ƿ�����ĺ��ʷ����� ��Ȼ��۲������жϡ�
A.���û����X�����ϲ���Һ���ٵ������Ȼ�����Һ B.����ҺY�еμ������Ȼ�����Һ
���ж������Ƿ�ϴ�Ӹɾ������Բ�ȡ������ϴ��Һ�еμ� ��Ȼ��۲������жϡ�
A.�Ȼ�����Һ B.ϡ���� C.̼������Һ D.ϡ����
�۸���ʵ�����ݣ�������Ʒ��̼���Ƶ���������Ϊ ��д��������̡�4�֣�
[Mr(BaCl2)=208 Mr(Na2CO3)=106 Mr(BaCO3)=197 ��Mr(NaCl)=58.5]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�꽭��ʡ�������������п���ѧ��ģ�Ծ��������棩 ���ͣ������
 NH3��+HCl����
NH3��+HCl����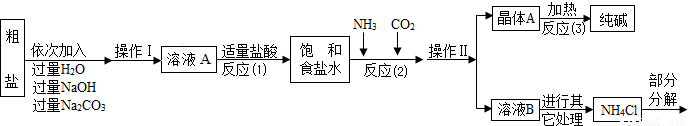
| ѡ���װ�� | ʵ������ | ʵ����� |
| ��Ʒ��������A |


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com