
 ��һ�ձ���ʢ��42.2g CaCO3��CaCl2�ķ�ĩ״���������м���188.8gˮ��ʹ������еĿ�������ȫ�ܽ⣮Ȼ������������μ������ʵ���������Ϊ10%��ϡ���ᣬ�ձ������ܹ������ʵ�������������ϡ�����������ϵ������ͼX��ʾ��
��һ�ձ���ʢ��42.2g CaCO3��CaCl2�ķ�ĩ״���������м���188.8gˮ��ʹ������еĿ�������ȫ�ܽ⣮Ȼ������������μ������ʵ���������Ϊ10%��ϡ���ᣬ�ձ������ܹ������ʵ�������������ϡ�����������ϵ������ͼX��ʾ��| 73 |
| 44 |
| 14.6g |
| x |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺
��һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
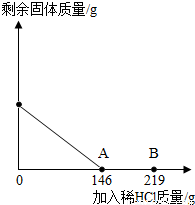
 ��2012?Ϋ����ģ����һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ����壨��ˮ�ܽ��ʣ����壩�����������ϡ�����������ϵ������ͼ��ʾ���������������������⣺
��2012?Ϋ����ģ����һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ����壨��ˮ�ܽ��ʣ����壩�����������ϡ�����������ϵ������ͼ��ʾ���������������������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?��ɽ��һģ����һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺
��2012?��ɽ��һģ����һ�ձ���ʢ��42.2g̼��ƺ��Ȼ��Ƶķ�ĩ״���������м���116.6gˮ��ʹ�Ȼ�����ȫ�ܽ⣮Ȼ����������μ���10%��ϡ���ᣬ�ձ���ʣ���������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�걱���з�ɽ���п���ѧһģ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com