
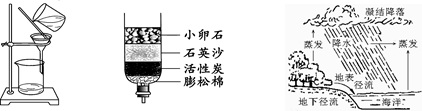


 ĆūĢā½š¾ķĻµĮŠ“š°ø
ĆūĢā½š¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŌŚŗÓĖ®ÖŠ¼ÓČėĆ÷·ÆæɳżČ„ĖłÓŠŌÓÖŹ |
| B£®Ģį³«³¤ĘŚÓĆ“æĖ®£ØÕōĮóĖ®£©ÉÕ²Ė”¢Öó·¹µČ |
| C£®Ė®ĢåÓŠ×Ō¾»ÄÜĮ¦£¬Ī“¾“¦ĄķµÄÉś»īĪŪĖ®æÉČĪŅāÅÅ·Å |
| D£®ŌŚµĖ®×ŹŌ“ȱ·¦µÄŗ£µŗÉĻ£¬æÉæ¼ĀĒÓĆÕōĮó·Ø“Óŗ£Ė®ÖŠĢįČ”µĖ® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ÓĆ·ŹŌķĖ®Ēų±šÓ²Ė®ŗĶČķĖ® | B£®ÓƵāĖ®Ēų±šµķ·ŪŗĶÕįĢĒ |
| C£®ÓĆ·ÓĢŖŹŌŅŗĒų±šŃĪĖįŗĶŹ³ŃĪĖ® | D£®ÓƵćČ¼·ØĒų±š¾ŪŅŅĻ©ŗĶ¾ŪĀČŅŅĻ© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
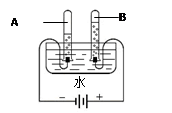 ¢Ś
¢Ś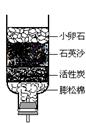 ¢Ū
¢Ū
 4X + Y”£ŌņXµÄ»ÆѧŹ½ĪŖ £¬YµÄ»ÆѧŹ½ĪŖ ”£
4X + Y”£ŌņXµÄ»ÆѧŹ½ĪŖ £¬YµÄ»ÆѧŹ½ĪŖ ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®×ŌČ»½ēÖŠµÄĖ®¶¼ŹĒ»ģŗĻĪļ | B£®Éś»īĪŪĖ®æÉČĪŅāÅÅ·Å |
| C£®Ė®ŹĒÉśĆü»ī¶Æ²»æÉȱɣµÄĪļÖŹ | D£®·ŹŌķĖ®æÉŅŌĒų·ÖČķĖ®ŗĶÓ²Ė® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
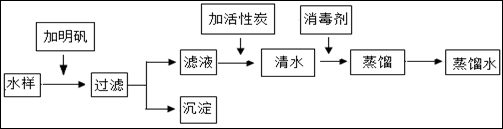

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com