
æĪĶā»ī¶ÆŠ”×éµÄĶ¬Ń§ŌŚ²ā¶ØÓÉNaClŗĶNa2CO3ŠĪ³ÉµÄ¹ĢĢå»ģŗĻĪļ×é³ÉŹ±£¬½ųŠŠĮĖŅŌĻĀŹµŃé£ŗČ”40 g¹ĢĢå»ģŗĻĪļÅä³ÉČÜŅŗ£¬Ę½¾ł·ÖĪŖĖÄ·Ż£¬Č»ŗó·Ö±š¼ÓČėŅ»¶ØÖŹĮæ·ÖŹżµÄCaCl2ČÜŅŗ£¬ŹµŃ鏿¾Ż¼ūĻĀ±ķ£ŗ
|
| ŹµŃéŅ» | ŹµŃ鶞 | ŹµŃéČż | ŹµŃéĖÄ |
| Ō¹ĢĢå»ģŗĻĪļÖŹĮæ | £±£°g | £±£°g | £±£°g | £±£°g |
| ¼ÓČėCaCl2ČÜŅŗÖŹĮæ | £±£°g | £²£°g | £³£°g | £“£°g |
| Éś³ÉµÄ³ĮµķµÄÖŹĮæ | £²g | £ķ | £µg | £µg |
Ēė·ÖĪö±ķÖŠŹż¾Ż»Ų“š²¢¼ĘĖć[£Ø4£©ŅŖĒ󊓼ĘĖć¹ż³Ģ ]
£Ø1£©Éś³ÉµÄ³ĮµķŹĒ£ØĢīŠ“»ÆѧŹ½£© ”£ £Ø2£©10gŌ¹ĢĢå»ģŗĻĪļÅä³ÉµÄČÜŅŗŗĶ×ćĮæCaCl2ČÜŅŗ·“Ó¦£¬×ī¶ąÉś³É³ĮµķÖŹĮæĪŖ g”£
£Ø3£©£ķ= g”£
£Ø4£©Ō¹ĢĢå»ģŗĻĪļÖŠNaClµÄÖŹĮæ·ÖŹżŹĒ¶ąÉŁ£æ
(1)CaCO3 £Ø1·Ö£© (2)5 £Ø1·Ö£© (3) 4 £Ø1·Ö£©
£Ø4£©CaCl2 + Na2CO3 = CaCO3”ż + 2NaCl
106 100
x 5g ””””””””””””””””””” £Ø1·Ö£©
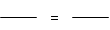 106 100
106 100
x 5g ””””””””””””””””””” £Ø1·Ö£©
x = 5.3g ””””””””””””””””””” £Ø1·Ö£©
¹ĢĢå»ģŗĻĪļÖŠNaCl µÄÖŹĮæ·ÖŹżŹĒ:
 10g- 5.3g
10g- 5.3g
10g ””””””” £Ø2·Ö £¬ĮŠŹ½ŗĶ½į¹ūø÷1·Ö£©
½āĪö:£Ø1£©Ö»ÓŠĢ¼ĖįøłĄė×ÓÓėøĘĄė×Ó»į²śÉś°×É«³ĮµķĢ¼ĖįøĘ£¬£Ø2£©ÓɱķæÉÖŖ10gŌ¹ĢĢå»ģŗĻĪļÅä³ÉµÄČÜŅŗŗĶ×ćĮæCaCl2ČÜŅŗ·“Ó¦£¬×ī¶ąÉś³É³ĮµķÖŹĮæĪŖ5g£¬£Ø3£©·ÖĪöŹµŃ鏿¾ŻæÉÖŖ£¬Ćæ¼ÓČė10gĀČ»ÆøĘČÜŅŗæÉÉś³É2g³Įµķ£¬Ņņ“Ė¼ÓČė20gĀČ»ÆøĘČÜŅŗŹ±£¬µĆµ½³ĮµķÖŹĮæm=2g”Į =4g£¬£Ø4£©¼ÓČėĀČ»ÆøĘČÜŅŗÖĮĶźČ«·“Ó¦Ź±£¬Éś³É³ĮµķÖŹĮæĪŖ5g£¬øł¾ŻĢ¼ĖįÄĘÓėĀČ»ÆøĘ·“Ó¦µÄ»Æѧ·½³ĢŹ½£¬æÉÓÉÉś³É³ĮµķĢ¼ĖįøʵÄÖŹĮæ¼ĘĖćÖŠŌ¹ĢĢå»ģŗĻĪļÖŠNa2CO3µÄÖŹĮ棬¼“æÉĒóµĆŌ¹ĢĢå»ģŗĻĪļÖŠNaClµÄÖŹĮ棬æÉĒóµĆŌ¹ĢĢå»ģŗĻĪļÖŠNaClµÄÖŹĮæ·ÖŹż£®
=4g£¬£Ø4£©¼ÓČėĀČ»ÆøĘČÜŅŗÖĮĶźČ«·“Ó¦Ź±£¬Éś³É³ĮµķÖŹĮæĪŖ5g£¬øł¾ŻĢ¼ĖįÄĘÓėĀČ»ÆøĘ·“Ó¦µÄ»Æѧ·½³ĢŹ½£¬æÉÓÉÉś³É³ĮµķĢ¼ĖįøʵÄÖŹĮæ¼ĘĖćÖŠŌ¹ĢĢå»ģŗĻĪļÖŠNa2CO3µÄÖŹĮ棬¼“æÉĒóµĆŌ¹ĢĢå»ģŗĻĪļÖŠNaClµÄÖŹĮ棬æÉĒóµĆŌ¹ĢĢå»ģŗĻĪļÖŠNaClµÄÖŹĮæ·ÖŹż£®


 ¾ŁŅ»·“ČżĶ¬²½Ēɽ²¾«Į·ĻµĮŠ“š°ø
¾ŁŅ»·“ČżĶ¬²½Ēɽ²¾«Į·ĻµĮŠ“š°ø æŚĖćÓėÓ¦ÓĆĢāæØĻµĮŠ“š°ø
æŚĖćÓėÓ¦ÓĆĢāæØĻµĮŠ“š°ø ĆūŹ¦µć¾¦×Ö“Ź¾ä¶ĪĘŖĻµĮŠ“š°ø
ĆūŹ¦µć¾¦×Ö“Ź¾ä¶ĪĘŖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ŹµŃéŅ» | ŹµŃ鶞 | ŹµŃéČż | ŹµŃéĖÄ | |
| Ō¹ĢĢå»ģŗĻĪļÖŹĮæ | 10g | 10g | 10g | 10g |
| ¼ÓČėCaCl2ČÜŅŗÖŹĮæ | 10g | 20g | 30g | 40g |
| Éś³ÉµÄ³ĮµķµÄÖŹĮæ | 2g | m | 5g | 5g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
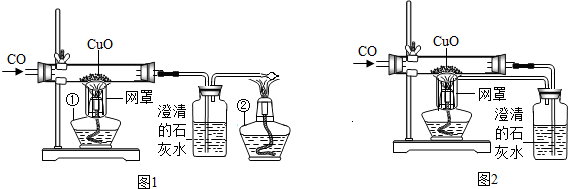
 ijŠ£æĪĶā»ī¶ÆŠ”×éµÄĶ¬Ń§ŌŚĄĻŹ¦µÄ°ļÖśĻĀ£¬Ģ½¾æÓĆ³ąĢśæó£ØÖ÷ŅŖ³É·ÖFe2O3£©Į¶ĢśµÄÖ÷ŅŖ·“Ó¦ŌĄķ£®ĖūĆĒÉč¼ĘµÄŹµŃé×°ÖĆ£¬ČēĶ¼£ŗ
ijŠ£æĪĶā»ī¶ÆŠ”×éµÄĶ¬Ń§ŌŚĄĻŹ¦µÄ°ļÖśĻĀ£¬Ģ½¾æÓĆ³ąĢśæó£ØÖ÷ŅŖ³É·ÖFe2O3£©Į¶ĢśµÄÖ÷ŅŖ·“Ó¦ŌĄķ£®ĖūĆĒÉč¼ĘµÄŹµŃé×°ÖĆ£¬ČēĶ¼£ŗ
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
| ||
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com