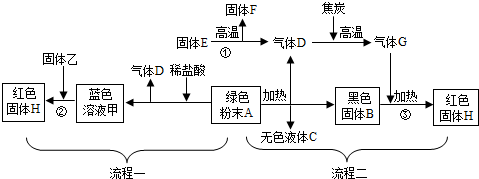
£Ø2012?Ģ©ÖŻ£©Ēė½įŗĻĻĀĮŠ³£ÓƵÄŅĒĘ÷ŗĶ×°ÖĆ£¬»Ų“šÓŠ¹ŲĪŹĢā£ŗ

£Ø1£©Š“³ö±źŗÅŅĒĘ÷µÄĆū³Ę£ŗb
׶ŠĪĘæ
׶ŠĪĘæ
£»e
ĮæĶ²
ĮæĶ²
£®
£Ø2£©ÉĻĶ¼ŅĒĘ÷ÖŠ£¬ÄÜŌŚ¾Ę¾«µĘ»šŃęÉĻÖ±½Ó¼ÓČȵÄÓŠ
c
c
£ØĢīŠņŗÅ£©£®
£Ø3£©Ń”ŌńÉĻĶ¼ÖŠµÄ
ab»ņac
ab»ņac
£ØĢīŠņŗÅ£©æÉ×éŗĻ³ÉŹµŃéŹŅÖĘČ”¶žŃõ»ÆĢ¼µÄ·¢Éś×°ÖĆ£¬ŌŚ²»Ģķ¼ÓĘäĖūŅĒĘ÷µÄĒéæöĻĀ£¬¼ģ²éøĆ·¢Éś×°ÖĆĘųĆÜŠŌµÄ·½·ØĪŖ
¹ŲÉĻÖ¹Ė®¼Š£¬Ļņ³¤¾±Ā©¶·ÖŠ×¢Ė®£¬ČōÄÜŠĪ³ÉĪČ¶ØµÄĖ®Öł£¬ŌņĘųĆÜŠŌĮ¼ŗĆ
¹ŲÉĻÖ¹Ė®¼Š£¬Ļņ³¤¾±Ā©¶·ÖŠ×¢Ė®£¬ČōÄÜŠĪ³ÉĪČ¶ØµÄĖ®Öł£¬ŌņĘųĆÜŠŌĮ¼ŗĆ
£»ŹÕ¼Æ¶žŃõ»ÆĢ¼ĘųĢ壬æÉŃ”ŌńÉĻĶ¼ÖŠµÄ
g
g
£ØĢīŠņŗÅ£©×°ÖĆ£»ČōŅŖ¼ģŃ鶞Ńõ»ÆĢ¼ĘųĢ壬ŌņŠč½«ĘųĢåĶعżŹ¢ÓŠ
³ĪĒåµÄŹÆ»ŅĖ®
³ĪĒåµÄŹÆ»ŅĖ®
µÄi×°ÖĆ£®
£Ø4£©ŌŚŹµŃéŹŅ³żČ„“ÖŃĪÖŠµÄ²»ČÜŠŌŌÓÖŹŹ±£¬³żÉĻĶ¼ÓŠ¹ŲŅĒĘ÷Ķā£¬±ŲŠėĢķ¼ÓµÄŅĒĘ÷²»°üĄØĻĀĮŠÖŠµÄ
CD
CD
£ØĢīŠņŗÅ£©£®
A£®²£Į§°ō B£®Õō·¢Ćó C£®Ė®²Ū D£®·ÖŅŗĀ©¶·£®




 ŹĄ¼Ķ°ŁĶØÖ÷ĢåæĪĢĆŠ”ѧæĪŹ±Ķ¬²½“ļ±źĻµĮŠ“š°ø
ŹĄ¼Ķ°ŁĶØÖ÷ĢåæĪĢĆŠ”ѧæĪŹ±Ķ¬²½“ļ±źĻµĮŠ“š°ø ŹĄ¼Ķ°ŁĶØÓÅĮ·²āĻµĮŠ“š°ø
ŹĄ¼Ķ°ŁĶØÓÅĮ·²āĻµĮŠ“š°ø °Ł·Öѧɜ×÷Ņµ±¾ĢāĮ·ĶõĻµĮŠ“š°ø
°Ł·Öѧɜ×÷Ņµ±¾ĢāĮ·ĶõĻµĮŠ“š°ø
 £Ø2012?Ģ©ÖŻČżÄ££©ĻÖÓŠĶ·ŪŗĶĆ¾·ŪµÄ»ģŗĻĪļѳʷ£¬Ä³ŠĖȤŠ”×éŅŖ²ā¶ØŃłĘ·ÖŠĆ¾µÄÖŹĮæ·ÖŹż£®ĖūĆĒ³ĘČ”øĆ»ģŗĻĪļѳʷ10gÖĆÓŚÉÕ±ÖŠ£¬Č»ŗó¼ÓČė350gČÜÖŹÖŹĮæ·ÖŹżĪŖ7.3%µÄĻ”ŃĪĖį£®Ėł¼ÓĻ”ŃĪĖįµÄÖŹĮæÓė²śÉśĒāĘųµÄÖŹĮæ¹ŲĻµČēĶ¼ĖłŹ¾£®
£Ø2012?Ģ©ÖŻČżÄ££©ĻÖÓŠĶ·ŪŗĶĆ¾·ŪµÄ»ģŗĻĪļѳʷ£¬Ä³ŠĖȤŠ”×éŅŖ²ā¶ØŃłĘ·ÖŠĆ¾µÄÖŹĮæ·ÖŹż£®ĖūĆĒ³ĘČ”øĆ»ģŗĻĪļѳʷ10gÖĆÓŚÉÕ±ÖŠ£¬Č»ŗó¼ÓČė350gČÜÖŹÖŹĮæ·ÖŹżĪŖ7.3%µÄĻ”ŃĪĖį£®Ėł¼ÓĻ”ŃĪĖįµÄÖŹĮæÓė²śÉśĒāĘųµÄÖŹĮæ¹ŲĻµČēĶ¼ĖłŹ¾£®