
ˮ�����������в���ȱ�ٵ����ʡ�
��1�����о�ˮ�����У�ͨ�����ڳ�ȥˮ�����������ʵ��� ����ˮ�̶���ߵ��� ��
| A������ | B����� | C������ | D������ |
��1��A��C ��2��4+ ��3��Ca��HCO3��2 ���� ��CaCO3 +H2O+CO2��
���������������1�����˿��Ѳ�����ˮ�����ʳ�ȥ��������Եõ�����������ˮ��ͨ�����ڳ�ȥˮ�����������ʵ��ǹ��ˣ���ˮ�̶���ߵ��������A��C����2���ڻ������У�Ԫ�ػ��ϼ۵Ĵ�����Ϊ�㡣�ڶ��������У���Ԫ�صĻ��ϼ���-2�ۣ����������Ԫ�صĻ��ϼ�Ϊ+4�ۡ����+4��
���㣺ˮ�ľ�������ѧʽ����д�����壻��ѧ����ʽ����д������Ԫ���볣��ԭ���ŵĻ��ϼۡ�


 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ɫ����A�ڼ��ȵ������·�Ӧ����B��C�Լ�D�������¹���������Һ����Cʱ�ֽ�ų�D������C�ڷ�Ӧǰ��������ͻ�ѧ���ʶ�û�иı䣬����ϸ��˿����D�е�ȼ�ܾ���ȼ�գ��������䣬���ɺ�ɫ����E��д�������ʵ����ƣ�A���������������� ������B���������������� ��C���������������� ������D���������������� ��E���������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ⶨ�������������������ʵ��װ������ͼ��һ����ʾ���ڼ���ƿ�ڼ���������ˮ����ȷ�ˮ�����ϵ��ݻ������ϼǺţ��ر�ֹˮ�У�ͨ��ʹ����ȼ�ա���ش��������⣺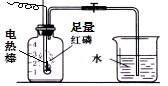

��ͼһ�� ��ͼ����
��1������ȼ�յ������� ��
��2������ȼ�յ����ֱ���ʽ ��������Ӧ���� ��
��3����ȴ���ɿ�ֹˮ�У���۲쵽������Ϊ ��
��4��ʵ����� ��
��5��ʵ�������������ֽ��뼯��ƿ��ˮ���������1/5��ԭ��
�������һ�㼴�ɣ�
��6������Ϩ�����ƿ��ʣ���������Ҫ�� ��
��7��ʵ��һװ�ñ�ʵ���װ�õ��ŵ��ǣ� �������һ�㼴�ɣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣���ѧ��ͨ����ˮ�����ɺ�ˮ�ķֽ�����ʶˮ����ɡ�����ͼ��
��1��ͨ��һ��ʱ����Թ�1��2�е����������Ϊ �����Թ�2�е缫�������ǵ�Դ�� �����������������
��2��ͨ��ֽ�ˮ�ķ��ű���ʽΪ ���ɴ˵ó����ۣ�ˮ�� ��ɣ�
��3���Թ��ڲ�����������п�ȼ�Ե��� ��ȼ�շ��� ���档
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ѧϰ�����Ļ�ѧ����ʱ���֣���ȼ��ȼ�յľ��ҳ̶���������Ũ���йأ������ȼ��������ĽӴ�����йء�ijʵ��С�����������ʵ�飬�벹������ʵ�鱨�档
| ʵ�� | ʵ�鲽�� | ʵ������ | �йط�Ӧ�Ļ�ѧ����ʽ |
| A | ȡ�������ȼ�����ʢ�п����ļ���ƿ��ȼ�� | �������ĵ���ɫ���� | ��1�� |
| ȡ�������ȼ�����ʢ�������ļ���ƿ��ȼ�� | ��2�� | ||
| B | ȡ����˿�պ�����ʢ�������ļ���ƿ��ȼ�� | ������ȼ�� | ��3�� |
| ȡϸ��˿�պ�����ʢ�������ļ���ƿ��ȼ�� | ����ȼ�գ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ʵ���ҿ�������װ����ȡ����ˮ������ش��������⡣
��1����ˮ��c������B�˽��룬�������ĺô��ǣ� ��
��2����ʵ��ǰ����������b�м��뼸�����Ƭ���������ǣ� ��
��3��d�����������ռ�����2mL~3mLҺ��Ҫ��ȥ��ԭ���ǣ� ��
��4�����ʵ��֤������d���ռ���ˮ����ˮ��
| ʵ�鲽�� | ʵ������ | ʵ����� |
| | | ��ˮ����ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼʾ�dz�����ˮ��������ͼ��
�Ķ�����ͼ���ش��������⣺
��1������ˮ���������У�һ������������ʹ�õĴ�����������_________�仯.
��2����������ˮ���г��ö��������������ʶ�ˮ�����������������ȵĻ�ѧʽ��_________��������Ԫ�صĻ��ϼ���_______��
��3����������������ˮ��Ϊ��ˮ���ɻ����ڳ�����ˮ�����֮һ��ˮ�к�������0.3���������ָ������ ��
��4������ˮ���������У�����̿�������� ��
��5���ճ��������㾭�����õĽ�ˮ��ʩ�У�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ˮ��ʵ���У���Դ�������������IJ������Ϸ�����������ֱ��� �� ���������ԼΪ �����ʵ��֤����ˮ���� ��ɵġ����ˮ�����ֱ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2013��4��20���Ĵ��Ű�����7.0��������������ˮ��ʳƷ��ȫ���ܹ�ע��
��1��ˮ�����ŵ���ζ�������ӻ���̿��ȥ�����������˻���̿���� ���ԣ�
��2�������������ˮ������ͨ���� ������������ˮ��Ӳ�ȣ�
��3����84����Һ���������������ߣ��Ʊ�����Ҫ�ɷִ������ƣ�NaClO���Ļ�ѧ����ʽΪ��
2NaOH+X=NaC1O+NaCl+H2O����X�Ļ�ѧʽΪ�� ����NaClO��Cl�Ļ��ϼ�Ϊ�� ����
��4����Ԯ������Ա��ȥ�İ�װʳƷ�У���һЩ����������˫���������ۼȿ��Է����ֿ��Ա���ʳƷ�������Ϊ���������տ����е��� ������ ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com