




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
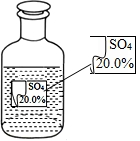 һ��Ȥζ��ѧ��У�����ʦ��ͬѧ��չʾ��һƿ��ǩ�������ɫ��Һ����ͼ��ʾ��Ҫ��ͬѧ�ǽ���̽����ȷ����ƿ��Һ������ʲô��Һ��
һ��Ȥζ��ѧ��У�����ʦ��ͬѧ��չʾ��һƿ��ǩ�������ɫ��Һ����ͼ��ʾ��Ҫ��ͬѧ�ǽ���̽����ȷ����ƿ��Һ������ʲô��Һ��| ���� | MgSO4 | Na2SO4 | ��NH4��2SO4 | H2SO4 |
| �ܽ�� | 35.1g | 19.5g | 75.4g | ��ˮ����Ȼ��� |
| ʵ����� | ʵ������ | ʵ����� |
| ��ȡ����Һ�������Թ��У������еμӼ��� |
��Һ���а�ɫ�������� | ����ٳ��� |
| ���ò�����պȡ����ԭ��Һ����pH��ֽˮ��������ɫ������ | ��ҺpHС��7 |
����۳��� |
| ʵ����� | ʵ������ | ʵ����� |
| ȡ����Һ�������Թ��У� |
����ܳ������÷�Ӧ�Ļ�ѧ����ʽΪ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?�����һģ��һ��Ȥζ��ѧ��У�����ʦ��ͬѧ��չʾ��һƿ��ǩ�������ɫ��Һ����ͼ��ʾ��Ҫ��ͬѧ�ǽ���̽����ȷ����ƿ��Һ������ʲô��Һ��
��2013?�����һģ��һ��Ȥζ��ѧ��У�����ʦ��ͬѧ��չʾ��һƿ��ǩ�������ɫ��Һ����ͼ��ʾ��Ҫ��ͬѧ�ǽ���̽����ȷ����ƿ��Һ������ʲô��Һ��| ���� | MgSO4 | Na2SO4 | ��NH4��2SO4 | H2SO4 |
| �ܽ�� | 35.1g | 19.5g | 75.4g | ��ˮ����Ȼ��� |
| ʵ����� | ʵ������ | ʵ����� |
| ��ȡ����Һ�������Թ��У������еμӼ��� NaOH�����������ƣ����𰸺������ɣ� NaOH�����������ƣ����𰸺������ɣ� ��Һ |
��Һ���а�ɫ�������� | ����ٳ��� |
| ���ò�����պȡ����ԭ��Һ����pH��ֽ�ϣ���������ɫ������ | ��ҺpHС��7 |
����۳��� |
| ʵ����� | ʵ������ | ʵ����� | ||||||||
| ȡ����Һ�������Թ��У� ���Թ��м�������NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿ� ���Թ��м�������NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
�д̼�����ζ����������� ��ɫʯ����ֽ���� �д̼�����ζ����������� ��ɫʯ����ֽ���� |
����ܳ������÷�Ӧ�Ļ�ѧ����ʽΪ ��NH4��2SO4+2NaOH
��NH4��2SO4+2NaOH
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
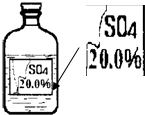 һ��Ȥζ��ѧ��У�����ʦ��ͬѧ��չʾ��һƿ��ǩ�������ɫ��Һ����ͼ��ʾ��
һ��Ȥζ��ѧ��У�����ʦ��ͬѧ��չʾ��һƿ��ǩ�������ɫ��Һ����ͼ��ʾ��| ���� | MgSO4 | Na2SO4 | ��NH4��2SO4 | H2SO4 |
| �ܽ�� | 35.1g | 19.5g | 75.4g | ��ˮ����Ȼ��� |
| ʵ����� | ʵ������ | ʵ����� |
| ��ȡ����Һ�������Թ��У������еμӼ��� NaOH NaOH ��Һ |
��Һ���а�ɫ�������� | ����ٳ��� |
| ���ò�����պȡ����ԭ��Һ����pH��ֽ�ϣ���������ɫ������ | ��ҺpHС��7 |
����۳��� |
| ʵ����� | ʵ������ | ʵ����� | ||||||||
| ȡ����Һ�������Թ��У� ���Թ��м�������NaOH��Һ�����ȣ� ���Թ��м�������NaOH��Һ�����ȣ� ��ʪ��ĺ�ɫʯ����ֽ�����Թܿ� ��ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
�д̼�����ζ����������� �д̼�����ζ����������� ��ɫʯ����ֽ���� ��ɫʯ����ֽ���� |
����ܳ������÷�Ӧ�Ļ�ѧ����ʽΪ ��NH4��2SO4+2NaOH ��NH4��2SO4+2NaOH
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 һ��Ȥζ��ѧ��У�����ʦ��ͬѧ��չʾ��һƿ��ǩ�������ɫ��Һ����ͼ��ʾ��Ҫ��ͬѧ�ǽ���̽����ȷ����ƿ��Һ��ʲô��Һ��
һ��Ȥζ��ѧ��У�����ʦ��ͬѧ��չʾ��һƿ��ǩ�������ɫ��Һ����ͼ��ʾ��Ҫ��ͬѧ�ǽ���̽����ȷ����ƿ��Һ��ʲô��Һ��| ���� | MgSO4 | Na2SO4 | ��NH4��2SO4 | H2SO4 |
| �ܽ�� | 35.1g | 19.5g | 75.4g | ��ˮ����Ȼ��� |
| ʵ����� | ʵ������ | ʵ����� |
| ��ȡ����Һ�������Թ��У������еμӼ��� NaOH NaOH ��Һ |
��Һ���а�ɫ�������� | ����ٳ��� |
| ���ò�����պȡ����ԭ��Һ����pH��ֽ�ϣ�������ɫ������ | ��ҺpHС��7 | ����۳��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
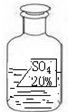 һ��Ȥζ��ѧ��У�����ʦ��ͬѧ��չʾ��һƿ��ǩ�������ɫ��Һ����ͼ��ʾ��Ҫ��ͬѧ�ǽ���̽����ȷ����ƿ��Һ������ʲô��Һ��
һ��Ȥζ��ѧ��У�����ʦ��ͬѧ��չʾ��һƿ��ǩ�������ɫ��Һ����ͼ��ʾ��Ҫ��ͬѧ�ǽ���̽����ȷ����ƿ��Һ������ʲô��Һ��| ���� | MgSO4 | Na2SO4 | ��NH4��2SO4 | H2SO4 |
| �ܽ�� | 35.1g | 19.5g | 75.4g | ��ˮ������Ȼ��� |
| ʵ����� | ʵ������ | ʵ����� |
| ��ȡ����Һ�������Թ��У������еμӼ��� NaOH NaOH ��Һ |
��Һ���а�ɫ�������� | ����ٳ��� |
| ���ò�����պȡ����ԭ��Һ����pH��ֽ�ϣ��ͱ�ɫ������ | ��Һ��pHС��7 | ����۳��� |
| ʵ����� | ʵ������ | ʵ����� |
| ȡ����Һ�������Թ��У� ����������NaOH��Һ��Ȼ��ʪ��ĺ�ɫʯ����ֽ�����Թܿ� ����������NaOH��Һ��Ȼ��ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
�д̼�����ζ�������������ɫʯ����ֽ���� �д̼�����ζ�������������ɫʯ����ֽ���� |
����ܳ������÷�Ӧ�Ļ�ѧ����ʽ ��NH4��2SO4+2NaOH= Na2SO4+2NH3��+2H2O ��NH4��2SO4+2NaOH= Na2SO4+2NH3��+2H2O |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com