����������Ƽ��������ȶ��벻����ѧ
��1����Դ��������������������ᷢչ������أ�
��Ŀǰ�������Ի�ʯȼ��Ϊ��Ҫ��Դ�������Ļ�ʯȼ�ϰ���ú��ʯ�ͺ�
��Ȼ��
��Ȼ��
��
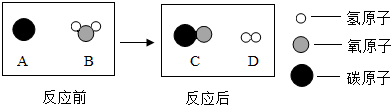
��Ϊ������Ⱦ�����ú�������ʣ��ɽ���ת��Ϊ��ȼ������ˮú�����˹��̿���Ϊ��̼��ˮ�ķ�Ӧ������ʾ��ͼ������ʾ��
�÷�Ӧ�Ļ�����Ӧ����Ϊ
�û���Ӧ
�û���Ӧ
���÷�Ӧ������ķ��Ӹ�����Ϊ
1��1
1��1
��
��ˮú���ڲ�ͬ���������£����Ժϳɲ�ͬ���ʣ�����Ҫ�л���״���CH
3OH����
д���÷�Ӧ�Ļ�ѧ����ʽ
��������Ϊ������ˮú���ڴ��������²��ܺϳ����ء�CO��NH
2��
2����������
��ѧ��Ӧǰ��Ԫ������䣬��Ӧ����û�е�Ԫ�أ��ʲ��ܵõ����أ�
��ѧ��Ӧǰ��Ԫ������䣬��Ӧ����û�е�Ԫ�أ��ʲ��ܵõ����أ�
��
��2�����ࡢ��֬�������ʶ�����������Ӫ�����ʣ�������ʳ�ǽ����Ļ�����֤��
��ʳ��ijɷ���Ҫ�е����ʡ�
����
����
����֬��ά���ء����κ�ˮ�������࣬ͨ����ΪӪ���أ�����ʳƷ�и��������ʵ���
BE
BE
����д��ţ���
A������ B������ C�������� D���� E��ţ��
�ڻƹ��и���ά����C��ά����C��pH��5�͵��µĻ����н��ȶ���Ϊ����ά����C����ʧ��ʳ�ûƹϵ���ѷ�����
�Ӵ�����
�Ӵ�����
��
�۴����еĵ��۷ֽ�����������պ��������ϸ���ĺ������ã�����������̼���ͷ�������ά�����������д����������һ�仯�Ļ�ѧ����ʽ
��
��3�����������������������Ҫ���ʻ�������������ܶ࣬ͨ���ɷ�Ϊ�������ϡ����ǽ������ϡ��߷��Ӻϳɲ��ϼ����ϲ��ϣ�
��������������ͭ���������ˡ����������������ǵ�
������
������
��������ʹ�������������������еĹ�ϵ�����������Լ����������ֽ�����̽�����ǵĽ������˳���ܴﵽĿ����
BC
BC
������ţ���
A����������Һ B������������Һ C������ͭ��Һ
�������йغϽ����ʵ�˵����ȷ����
D
D
������ĸ����
A���Ͻ���۵�һ������ijɷֽ����� B���Ͻ��Ӳ��һ������ijɷֽ�����
C����ɺϽ��Ԫ��������ͬ���Ͻ�����ܾ�һ����ͬ
D���Ͻ�����ɷֽ�����ȣ�����������������������ѧ���е����
�۸�����
���Ͻ�
���Ͻ�
������Ͻ𡱻����Ͻ𡱣���
�����жԽ�����Ʒ��ȡ�ķ�����������ȷ����
C
C
������ţ���
A���ڵ��ߵ��������һ�����ϲ� B�������г���Ȧ�϶���һ�������C���ں��ֵ���������Ϻ���ͭ
�ݺϳ����ϡ��ϳ���
�ϳ���ά
�ϳ���ά
�dz�˵������ϳɲ��ϣ��������ںϳ�����
A
A
����ĸ����
A��������̥ B������ C��������ϩ��Ʒ D�������֣�




